|
Case Report
Recurrent sialoblastoma of the parotid and submandibular glands: A case report and review
1 MD, PhD, Associate Professor of Pediatric Surgery, Surgery Department, Medicine School, Federal University of Rio de Janeiro (UFRJ), Rio de Janeiro, Brazil
2 Pediatric Surgery Division, Instituto de Puericultura e Pediatria Martagão Gesteira (IPPMG), Federal University of Rio de Janeiro (UFRJ), Rio de Janeiro, Brazil
3 MD, PhD, Pediatric Surgery Division, Instituto de Puericultura e Pediatria Martagão Gesteira (IPPMG), Federal University of Rio de Janeiro (UFRJ), Rio de Janeiro, Brazil
4 MD, Pediatric Surgery Division, Instituto de Puericultura e Pediatria Martagão Gesteira (IPPMG), Federal University of Rio de Janeiro (UFRJ), Rio de Janeiro, Brazil
5 MD, PhD, Full Professor of Pathology, Department of Pathology, Medicine School, Federal University of Rio de Janeiro (UFRJ), Rio de Janeiro, Brazil
6 MD, PhD, Adjunct Professor of Pathology, Department of Pathology, Medicine School, Federal University of Rio de Janeiro (UFRJ), Rio de Janeiro, Brazil
Address correspondence to:
Ivonete Siviero
MD, PhD, Associate Professor of Pediatric Surgery, Surgery Department, Medicine School, Federal University of Rio de Janeiro (UFRJ), Rio de Janeiro,
Brazil
Message to Corresponding Author
Article ID: 101498Z01IS2025
Access full text article on other devices

Access PDF of article on other devices

How to cite this article
Siviero I, Simões BCB, Méio IB, Sciani DC, Madi K, Chagas VLA. Recurrent sialoblastoma of the parotid and submandibular glands: A case report and review. Int J Case Rep Images 2025;16(1):48–53.ABSTRACT
Introduction: Sialoblastoma is a rare malignant embryonal tumor derived from primitive cells of the salivary glands. It occurs predominantly in the parotid gland and in the neonatal period. In this study, we present a case of sialoblastoma affecting the submandibular and parotid glands and review the literature on the subject.
Case Report: A 19-day-old newborn was presented with a history of a rapidly growing cervical mass since birth. A computed tomography (CT) scan showed a tumor in the left parotid and submandibular glands. He underwent tumor excision at 29 days of age. He had two post-operative recurrences, at 12 and 16 weeks of age. The diagnosis was made by histopathological examination. The tumor involved the facial nerve, and complete resection of the nerve only stopped recurrences after neural excision. The patient has remained relapse free for 18 years but has facial paralysis.
Conclusion: Sialoblastoma should be considered in the differential diagnosis of childhood facial tumors. Despite early diagnosis and extensive resection, there is a propensity for aggressive local recurrences. Neural invasion, capsular rupture, and Ki-67 positivity in immunohistochemical tests are factors that may be decisive in tumor recurrence.
Keywords: Parotid tumor, Salivary malignancy, Sialoblastoma, Submandibular gland
Introduction
Sialoblastoma is a very rare embryonal tumor that originates in the epithelial cells of the salivary glands [1]. First described in 1966 as an “embryoma” due to its occurrence in the fetal period by Vawter and Tefft [2], it was renamed sialoblastoma in 1988 by Taylor. The term refers to a neoplasm originating from the proliferation of immature or blastematous cells, imitating the development of the salivary gland but without constituting the acinar pattern [3]. Although it has a low degree of malignancy, since 2005 it has been classified as a malignant tumor by the World Health Organization (WHO) [4]. Although sialoblastoma is defined as a low-grade malignant neoplasm of the salivary glands in the WHO classification of head and neck tumors, its histology, genetics, and behavior remain controversial due to its rarity. It can present genetic mutations or morphological alterations that increase the risk of recurrence and metastasis [5].
It is estimated that 2–5% of all salivary gland tumors occur in children. Sialoblastoma has an annual incidence of 0.8 per million, making it exceptionally rare [1],[6]. They predominantly arise in the parotid region [6],[7]. It is locally invasive, aggressive, and, rarely, can metastasize to the lymph nodes or lungs if left untreated [1],[7]. It is most common in the first year of life, especially in the neonatal period. It is rarely diagnosed in the antenatal period or late in childhood. There are just over 60 cases described in the English literature to date, most of them as case reports [6]. It consists of basaloid epithelial cells, ductal structures, and myoepithelial cells, which form clusters surrounded by connective tissue.
We present the report of a patient with sialoblastoma of the submandibular and parotid glands, with two recurrences in three months. We also discuss the immunohistopathological study of the neoplasm, its evolution, and emphasize the need for early diagnosis and treatment due to the high rate of local recurrence.
Case Report
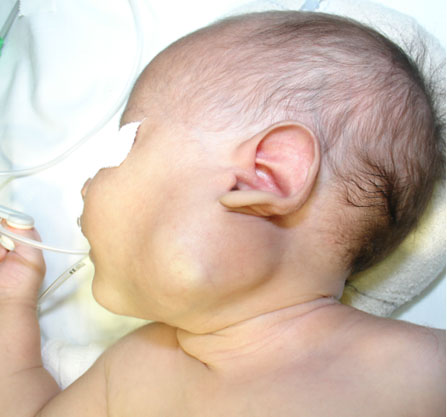
A male newborn was seen at the age of 19 days, with a history of a left cervical tumor since birth, which was progressive and fast-growing. On examination, there was a mass measuring approximately 5 cm in the left cervical region, submandibular, which obliterated the angle of the mandible, immobile, with a hard consistency (Figure 1).
A cervical computed tomography (CT) scan showed a voluminous, heterogeneous, expansile formation with dense foci in between, heterogeneous impregnation by the contrast medium, and non-contrasted hypodense areas with a density of fluid, in the left parotid space, receding medially into the carotid and pharyngeal spaces. The left parotid and submandibular glands were not individualized, and the left jugular vein was compressed by the mass. The right parotid and submandibular glands showed no alterations. Computed tomography scans of the skull, chest, and abdomen were normal. He underwent tumor excision at 29 days of age. During surgery, an irregular, lobulated mass was observed next to the carotid artery and the left internal jugular vein, completely involving the terminal branch of the facial nerve and adhering to the posterior muscle planes, where it showed a rupture of its tenuous capsule (Figure 2).
A 1.8 × 1.0 × 0.5 cm lymph node was also excised, which showed reactive follicular hyperplasia and no malignancy. He progressed well, with no signs of recurrence, and was assessed weekly. At 12 weeks of age, he presented with a local recurrence and was operated on at 13 weeks and underwent a second resection. It recurred in the deep scar area and underwent a third resection (2nd recurrence) at 16 weeks of age (112 days old) (Figure 3).
In the last surgery, a total left parotidectomy was performed, including the facial nerve, skin excision of the segment attached to the mass and excision of the superficial and deep cervical chain lymph nodes. Microscopic analysis confirmed the diagnosis of recurrent sialoblastoma and the absence of neoplasia in the skin, subcutaneous cellular tissue, and lymph nodes. Initially, the neoplasm appeared to be only in the submandibular gland, but in the second recurrence, it was noted that it originated in the parotid gland and extended to the submandibular gland. It evolved with facial paralysis, and he has not recurred for 18 years.
On macroscopy, the original mass was 4.5 × 3.5 × 3.5 cm; it was lobulated, light brown, covered by a fibrous capsule, with a rotated area, and with a whitish, solid cut surface septate by yellowish-white beams. First recurrence: irregular mass measuring 1.8 × 1.2 cm; cut, whitish, uniform, apparently encapsulated. Second recurrence: nodular formation measuring 4 × 4 × 2.5 cm, lobulated, pinkish-brown and covered by a thin capsule; on section, with a white area, with clear and regular boundaries, 2.5 cm in diameter.
Microscopy, hematoxylin/eosin (HE) and periodic acid-Schiff (PAS) staining showed a neoplasm of round embryonic epithelial cells, with amphophilic cytoplasm, sometimes vacuolized and ovoid or round nuclei with evident nucleoli, occasional mitoses, and frequent apoptosis. The cells were arranged in nodules and tubular structures, isolated by a connective stroma. There were also areas of necrosis and focal interruption of the fibrous capsule surrounding the neoplasm. In the preserved parenchyma of the parotid gland, multiple bulky intranuclear or tiny intracytoplasmic basophilic inclusions were seen in ductal cells, compatible with cytomegalovirus infection. Immunohistochemistry revealed the presence of basaloid and ductal cells positive for cytokeratin, S-100 protein, and Ki-67 (Figure 4).
The first surgery and the first recurrence showed focal interruption of the fibrous capsule, which was not seen in the second recurrence. The other histopathological signs were repeated in the three surgeries.
Written informed consent for publication was obtained from the patient’s legal guardian. Institutional ethical approval was granted by the Research Ethics Committee of the Martagão Gesteira Institute of Child Care and Pediatrics (IPPMG) of the Federal University of Rio de Janeiro (UFRJ), under number 6.973.926.
Discussion
Sialoblastoma is a rare tumor that arises from the primitive ductal epithelium of the salivary glands [1]. Lesions can be detected at gestational ultrasound (US) in less than 10% of cases [5]. In most cases it will be noticed at birth or in the first few weeks of life, as described in this case. There have been reports of slow-growing tumors that manifested late in childhood [1]. There is no difference in incidence between the sexes or racial predilection [1],[6].
Most of the time it originates in the parotid gland and in 10–15% it appears in the submandibular, sublingual, and minor salivary glands [8]. Clinically, it manifests as a hard mass in the parotid or submandibular region, which can vary in size from a small tumor to very large ones. Generally, the skin is not affected, but there have been reports of ulceration and extracutaneous growth noted at birth, in a case where the cervicofacial tumor was observed on gestational USG6 and of superficial hemorrhage, necrosis, cutaneous hamartoma and associated nevi [6],[9],[10]. In the case study presented in this paper, the tumor affected the left submandibular gland and the distal segment of the left parotid gland and, in the recurrence, although the macroscopic aspect of the skin suggested involvement, this was not confirmed in the histopathological result. Nor did it affect the regional lymph nodes.
There are reports of an association between sialoblastoma and hepatoblastoma in the literature [1],[6],[11],[12]. It is known that hepatoblastoma and sialoblastoma are congenital tumors that occur due to the disordered proliferation of a primitive organ blastema. Siddiqi et al. suggested that this association may occur due to an abnormality in a progenitor cell line [12].
The imaging characteristics of sialoblastoma have been documented in a limited number of cases. The CT appearance is of a soft tissue mass that is hypodense to the brain and isodense to the muscle [10],[13]. On magnetic resonance imaging (MRI), it can present with low and intermediate signal intensity when compared to the brain on T1 (T1-W) and T2 (T2-W), respectively. When there is greater hyperintensity on T2-W images, it suggests a high nucleus/cytoplasm ratio belonging to the blastoma and can be predictive of the diagnosis of blastomas [9],[11],[13]. Hemangiomas usually show hypervascularization on color Doppler, uniform, and intense enhancement after contrast injection on CT and MRI, and the presence of flow voids on spin-echo MRI [14]. Thus, MRI is the only imaging exam that can show predictive images for sialoblastoma, but no imaging exam can establish the etiological diagnosis of this mass.
The diagnosis can only be established with a histopathological examination of the mass. Currently, fine-needle aspiration biopsy (FNAB) has been applied with good accuracy in diagnosing potential malignancy risks in salivary gland tumors [6],[7], but the gold standard is still histopathological analysis of the excised mass.
Sialoblastomas are mitotically active masses of primitive cells, with formative ducts and pseudo-ductular spaces without acinar differentiation. Their appearance resembles the pre-acinar stages of salivary gland morphology [15].
Immunohistopathological findings characterized the neoplasm as a sialoblastoma, whose main differential diagnoses are congenital basal cell adenoma, monomorphic basaloid adenoma, embryonal carcinoma, and adenoid cystic carcinoma [7]. The main histological and immunohistochemical differences between these tumors are illustrated in Table 1 [16],[17],[18],[19],[20].
The high proliferative index (pronounced Ki-67 positivity), the presence of necrosis, mild cellular atypia, the infiltrative pattern, and recurrence make it difficult to classify this neoplasm as benign or malignant. Despite being classified as malignant since 2005 by the WHO, there is controversy about its malignant behavior in literature. As they are mitotically active and may show necrosis in some cases, this suggests malignant potential. However, it is believed to have low malignant potential, a high rate of local recurrence, and a low risk of metastasis [7]. Batsakis et al. proposed histological criteria for assessing malignancy in a sialoblastoma, which included invasion of nerves or vascular spaces and ancillary findings of necrosis and cytological atypia beyond that expected or presumed for an embryonic epithelium [21]. Mitotic figures are variable and more numerous in recurrent cases, as are areas of necrosis and total tumor replacement of the tumor stroma [9].
Ki-67 immunostaining can also be useful as a histological and prognostic indicator of these patients’ progression to recurrence or metastasis. Proliferative indices are lower in favorable cases and significantly higher in unfavorable tumors [22].
Complete surgical excision with tumor-free margins is still the main stage of primary treatment. These tumors are locally invasive and have a propensity to recur even after complete excision. Although metastases are infrequent, there have been reports of metastases to the lymph nodes and lungs [1],[7]. Whenever possible, the facial nerve should be preserved [6]. There is an increased likelihood of recurrence due to invasion of the neural sheath [6],[9],[23], as was noted in this case. While the patient did not undergo radical parotidectomy and facial nerve resection, he persisted with recurrences. In addition, there was a rupture of the tenuous capsule during surgical excision, which cannot be visualized macroscopically during the procedure. The capsule, as well as not being visible, may not be present [6]. The number of recurrences can vary from 1 or 2 to multiple. In this case report, the factors that probably directly influenced the recurrence were neural invasion, capsular rupture, and Ki-67 positivity. Recurrences can occur early, as reported in this patient, or a few years later, and long-term follow-up is essential [1],[6],[22].
Although early surgical resection with tumor-free margins is the first line of management, there is no consensus on the best course of treatment for aggressive tumors and on the efficacy of using neoadjuvant therapy, such as chemotherapy and radiotherapy. As sialoblastoma is very rare, consensus on the chemotherapy regimen is limited. Some studies and case reports refer to the use of vincristine (1.5 mg/m2 per week), cyclophosphamide (500–750 mg/m2 every three weeks), and doxorubicin (60–75 mg/m2 every three weeks), i.e., the chemotherapy drugs used in salivary neoplasms. Although specific response rates for sialoblastomas are not well documented, some reports suggest partial or complete responses in cases where chemotherapy was used as an adjuvant to surgery or for metastatic disease [1],[10],[13]. Other authors point out that sialoblastoma tends to respond well to chemotherapy and that its use would avoid extensive surgery, which can be mutilating and leave aesthetic and nervous system sequelae [11],[14],[24].
What can be said is that due to the rarity of this tumor, it is very difficult to assess its biological behavior and prognosis and that due to its aggressive nature, it is important to diagnose and treat these lesions early.
Conclusion
Sialoblastoma, a rare salivary gland tumor, should be included in the differential diagnosis of childhood facial tumors. Despite early diagnosis and extensive resection, it has a high potential for aggressive local recurrences.
REFERENCES
1.
Irace AL, Adil EA, Archer NM, Silvera VM, Perez-Atayde A, Rahbar R. Pediatric sialoblastoma: Evaluation and management. Int J Pediatr Otorhinolaryngol 2016;87:44–9. [CrossRef]
[Pubmed]

2.
Vawter GF, Tefft M. Congenital tumors of the parotid gland. Arch Pathol 1966;82(3):242–5.
[Pubmed]

3.
Taylor GP. Congenital epithelial tumor of the parotid-sialoblastoma. Pediatr Pathol 1988;8(4):447–52. [CrossRef]
[Pubmed]

4.
Seethala RR, Stenman G. Update from the 4th Edition of the World Health Organization Classification of Head and Neck Tumours: Tumors of the Salivary Gland. Head Neck Pathol 2017;11(1):55–67. [CrossRef]
[Pubmed]

5.
Jia X, Leng N, Wang M, Zhan X, Li J. Sialoblastomas with solid pattern have FGFR2 mutations and an unfavorable prognosis. Am J Surg Pathol 2025;49(4):336–46. [CrossRef]
[Pubmed]

6.
Cruz VMS, Pérez-de-Oliveira ME, Dos Santos Leite ÉG, et al. Clinicopathological study and survival outcomes of sialoblastoma: A systematic review. Head Neck 2023;45(8):2136–48. [CrossRef]
[Pubmed]

7.
Garrido A, Humphrey G, Squire RS, Nishikawa H. Sialoblastoma. Br J Plast Surg 2000;53(8):697–9. [CrossRef]
[Pubmed]

8.
Kataria SP, Kumar S, Singh G, Kalra R, Sen R, Garg N. Sialoblastoma. Diagnosis by FNAC: A case report. Diagn Cytopathol 2015;43(11):924–7. [CrossRef]
[Pubmed]

9.
Choudhary K, Panda S, Beena VT, Rajeev R, Sivakumar R, Krishanan S. Sialoblastoma: A literature review from 1966–2011. Natl J Maxillofac Surg 2013;4(1):13–8. [CrossRef]
[Pubmed]

10.
Opiła R, Feszak S, Wawryków P, Peregud-Pogorzelski J. Infant with parotid sialoblastoma and nevus sebaceous, treated with surgery and adjuvant chemotherapy. Children (Basel) 2023;10(4):628. [CrossRef]
[Pubmed]

11.
Rodríguez-Zubieta M, Albarenque K, Lagues C, et al. Two synchronous neonatal tumors: An extremely rare case. Case Rep Pathol 2021;2021:6674372. [CrossRef]
[Pubmed]

12.
Siddiqi SH, Solomon MP, Haller JO. Sialoblastoma and hepatoblastoma in a neonate. Pediatr Radiol 2000;30(5):349–51. [CrossRef]
[Pubmed]

13.
Wang L, Chen WL, Chen JF, Pan CB, Zhao XP. Surgical excision of sialoblastoma in the parotid gland in newborn. Int J Pediatr Otorhinolaryngol 2013;77(8):1268–71. [CrossRef]
[Pubmed]

14.
Saribeyoglu ET, Devecioglu O, Karakas Z, et al. How to manage an unresectable or recurrent sialoblastoma. Pediatr Blood Cancer 2010;55(2):374–6. [CrossRef]
[Pubmed]

15.
Tatlidede S, Karsidag S, Ugurlu K, Sadikoglu B, Tanik C, Bas L. Sialoblastoma: A congenital epithelial tumor of the salivary gland. J Pediatr Surg 2006;41(7):1322–5. [CrossRef]
[Pubmed]

16.
Zhu S, Schuerch C, Hunt J. Review and updates of immunohistochemistry in selected salivary gland and head and neck tumors. Arch Pathol Lab Med 2015;139(1):55–66. [CrossRef]
[Pubmed]

17.
Yang R, Zhan Y, Li Y, et al. The cellular and molecular landscape of synchronous pediatric sialoblastoma and hepatoblastoma. Front Oncol 2022;12:893206. [CrossRef]
[Pubmed]

18.
19.
Nagao T, Sato E, Inoue R, et al. Immunohistochemical analysis of salivary gland tumors: Application for surgical pathology practice. Acta Histochem Cytochem 2012;45(5):269–82. [CrossRef]
[Pubmed]

20.
21.
Batsakis JG, Mackay B, Ryka AF, Seifert RW. Perinatal salivary gland tumours (embryomas). J Laryngol Otol 1988;102(11):1007–11. [CrossRef]
[Pubmed]

22.
Williams SB, Ellis GL, Warnock GR. Sialoblastoma: A clinicopathologic and immunohistochemical study of 7 cases. Ann Diagn Pathol 2006;10(6):320–6. [CrossRef]
[Pubmed]

23.
Saravakos P, Hartwein J, Fayyazi A. Sialoblastoma of the parotid gland in a 13-year-old girl with multiple recurrences and long-term follow-up. Head Neck 2016;38(1):E13–5. [CrossRef]
[Pubmed]

24.
Prigent M, Teissier N, Peuchmaur M, et al. Sialoblastoma of salivary glands in children: Chemotherapy should be discussed as an alternative to mutilating surgery. Int J Pediatr Otorhinolaryngol 2010;74(8):942–5. [CrossRef]
[Pubmed]

SUPPORTING INFORMATION
Author Contributions
Ivonete Siviero - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Betina Carla Bertrand Simões - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ivens Baker Méio - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Deborah Câmara Sciani - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Kalil Madi - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Vera Lucia Antunes Chagas - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Guarantor of SubmissionThe corresponding author is the guarantor of submission.
Source of SupportNone
Consent StatementWritten informed consent was obtained from the patient for publication of this article.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Conflict of InterestAuthors declare no conflict of interest.
Copyright© 2025 Ivonete Siviero et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.