|
Case Report
Dapsone-induced Methemoglobinemia in a patient with history of Lichen Planus: A case report
1 Department of General Medicine, GMERS Medical College and Hospital, Sola, Ahmedabad, Gujarat, India
Address correspondence to:
Nandini Kaushik Dave
Department of General Medicine, GMERS Medical College and Hospital, Sola, Ahmedabad, Gujarat,
India
Message to Corresponding Author
Article ID: 101497Z01ND2025
Access full text article on other devices

Access PDF of article on other devices

How to cite this article
Dave NK. Dapsone-induced methemoglobinemia in a patient with history of lichen planus: A case report. Int J Case Rep Images 2025;16(1):43–47.ABSTRACT
This is a case of a 51-year-old patient with a history of lichen planus on dapsone having methemoglobinemia, with G6PD blood levels that are on the lower range. The primary focus of the case report is on the mechanism of dapsone-induced methemoglobinemia along with a discussion over methemoglobinemia, its signs and symptoms, diagnosis, and the line of treatment for the same. Methemoglobinemia could be fatal if left undiagnosed and not treated on time.
Keywords: Dapsone, G6PD enzyme, Half life, Methemoglobinemia, Methylene blue, Nikolsky sign, Vitamin C
Introduction
Methemoglobinemia is a rare and potentially life-threatening condition characterized by the decreased oxygen-carrying capacity of hemoglobin due to the conversion of iron from the reduced ferrous (Fe2+) state to the oxidized ferric (Fe3+) state, which makes it incapable of binding oxygen molecules. Cyanosis occurs when 10–25% of the total hemoglobin turns into methemoglobin. Methemoglobinemia can be congenital; however, most common causes, though rare, are acquired due to chemical or topical agents. In our case, it was dapsone. If a patient’s history confirms exposure to an oxidative agent, the patient should be treated immediately to prevent adverse outcomes. Due to the rarity of this condition, it is important for clinicians to recognize the symptoms and offending agents of methemoglobinemia for prompt diagnosis and treatment. Here, we report the diagnosis and management of a patient with dapsone-induced methemoglobinemia with evident cyanosis.
Case Report
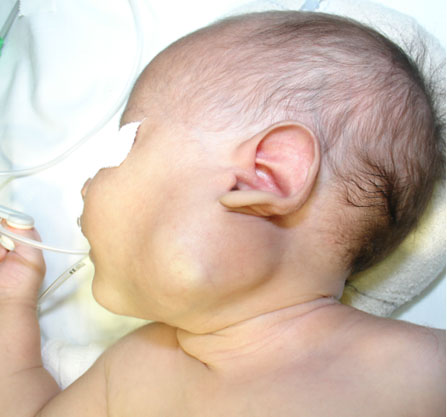
The patient is a 51–year-old Indian female with a history of lichen planus (LP) (1.5 years). She was on topical steroids until 10 days before her hospital arrival, when she started dapsone (100 mg OD) for the same condition. Lichen planus is a condition which primarily affects the mucosal layers. The patient had lesion in the buccal mucosa that was whitish and reticular with a positive Nikolsky sign. There were no other co-morbid conditions present and neither a significant past history except for a case of dengue eight years back wherein the patient needed hospitalization for three days (platelet count went till 90,000 cells/dL). The patient came with complaints of breathlessness without exertion and shortness of breath. The saturation measured using Pulse oximeter in the outpatient department (OPD) showed 84%. The patient was tachycardic, the pulse rate being 119/min, respiration 26 breaths/min (associated with shortness of breath), and had normal body temperature. The patient was conscious, cooperative, and well oriented to time, place, and person. The general physical examination showed signs of peripheral cyanosis (i.e., a faint bluish discoloration of the skin of lips and the extremities) (Figure 1). There were no signs of lymphadenopathy, clubbing, pedal edema, icterus, or pallor.
The patient was shifted to the Emergency Room for stabilization and further testing. Oxygen supplementation was immediately started with nasal prongs and the level of O2 being 8 L. Keeping a record the saturation now ranged from 90% to 92% saturation. An electrocardiogram (ECG) showed normal rate, rhythm, and waveforms. A 2D ECG showed an ejection fraction (EF) of 65%, with trivial aortic stenosis and aortic regurgitation. The rest of the findings were nonsignificant [no abnormality detected (NAD)]. The ECG was done to rule out any cardiac etiology causing the symptoms (Table 1).
The arterial blood gas analysis values showed that the patient was suffering from respiratory alkalosis (hence the hyperventilation), where the most common reasons were hemolytic anemia and a compensatory metabolic acidosis. Blotting paper test changed the blood color to its darker shade in room air, which indicated a possibility of methemoglobinemia. After doing the hematology test for the same the level of methane in blood was found to be 10.3% of the total. Glucose-6-phosphate dehydrogenase enzyme (G6PD) and haptoglobin levels were examined and the former was 4.61 (at the normal lower limit) and the latter was 7.02 (indicating the presence of hemolytic anemia as well). The patient was shifted to the intensive care unit (ICU) after being admitted for a day to be kept under observation. The oxygen saturation stayed between 91% and 93% with oxygen mask at 8 L O2. The patient was vitally stable, here pulse rate was 98/min, blood pressure (BP) was 106/59 mmHg, respiratory rate (RR) was 15/min, normal jugular venous pressure (JVP) also noted. The patient was administered normal saline (NS) pint + multivitamin injection was also substituted. All the routine laboratory examinations were normal. The lactate dehydrogenase (LDH) levels were slightly elevated 271 U/L as well as the reticulocyte count: 3.8%. The medications given to the patient were Folvite, Limcee (Vitamin C), and Ranitidine.
Methylene blue is the standard line of therapy for a patient suffering from hemolytic anemia but this patient was refrained from the same because of already low levels of G6PD enzyme, which is needed in sufficient amount for methylene blue to work out its function. The patient’s condition improved (Figure 2) and signs of peripheral cyanosis diminished after a day and with oxygen therapy. The saturation remained at a constant 95% at 4 L O2 with nasal prongs, so the patient was shifted to the room. She was kept under observation in the hospital for two days, during which time the drug would likely be mostly cleared from her system, allowing her oxygen saturation to return to normal. The symptoms had declined and there was no breathlessness. The patient was discharged with O2 saturation: 97% in room air, pulse: 84/min, BP: 110/64 mmHg, and RR: 17 breaths/min.
The patient was prescribed multivitamin capsules and was advised not to take drugs causing hemolytic anemia (sulpha class, primaquine, chloroquine, nitrofurantoin) and also to avoid exposure to aniline dyes.
Discussion
Lichen planus
Lichen planus (LP) is an autoimmune condition wherein the body’s immune system attacks its own and the cause is idiopathic but maybe caused by a genetic predisposition, stress, localized skin disease like herpes zoster and sometimes in graft-vs-host disease. Its incidence lies majorly between ages 30 and 60 years.
The clinical features of lichen planus are:
- Cutaneous LP majorly causes “6Ps”: Purple papules, pruritic, planar (flat), polygonal, and plaques. It is the most distinctive kind of LP usually found over the wrists and shins as well as the sun exposed areas.
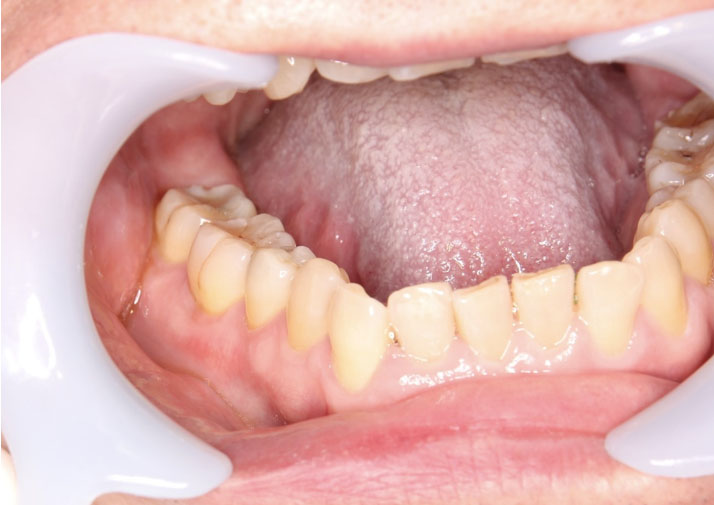
- Oral LP is painless white reticular streaks of mucosa (Wickham striae) which could be peeled off with gentle pressure (positive Nikolsky sign). If more severe it may cause painful erosive ulcers.
- Vulval LP causes lacy fern like pattern over the mucosa, if advanced it may cause erosive painful ulcers and desquamation. Other less common sites include penile shaft, larynx, anus, nails, and other mucosal surfaces. Lichen planus is generally diagnosed with patch testing. Histology shows wedge-shaped hypergranulosis (saw tooth rete ridges) between the dermal and the epidermal layers. This patient only had oral LP which is painless with the reticular streaks present.
- The line of treatment is always topical (steroids and emollients), if still it persists oral therapy should be started (immunosuppressant, steroids, dapsone, and antihistaminics) (Figure 3).
Glucose-6-phosphate dehydrogenase enzyme (G6PD) deficiency
G6PD enzyme is found in the cytoplasm of cells. Its main function is to prevent oxidative damage the cell from caused by reactive oxygen species. It is majorly found in red blood cells, thus preventing its disintegration when faced with an oxidative injury. This patient has the enzyme level on the lower side of normal (4.61 MEq/L), thus, when exposed to drugs like dapsone, which has a tendency to form reactive oxygen species (ROS), the red blood cells (RBCs) cannot be protected from the damage and may cause hemolytic anemia as well as methemoglobinemia. Other classes of drugs may also cause the same symptoms like antimalarials (primaquine), nitrofurantoins, sulphadiazines, and aniline dyes as well. Its mechanism is explained for the action of methylene blue.
Dapsone indications and metabolism
Dapsone is a sulfone antibiotic and anti-inflammatory agent that inhibits folate synthesis [1]. It has traditionally been used against leprosy, but in the modern era, it has been prescribed for dermatological conditions including pyoderma gangrenosum, dermatitis herpetiformis, LP, and several infectious organisms like Pneumocystis jirovicii (PJP) and toxoplasmosis [1],[2],[3].
The metabolism of dapsone includes two routes: nitrogen (N)-acetylation and N-hydroxylation. The former is considered the major route of dapsone metabolism, forming mono-acetyl dapsone and di-acetyl dapsone where the N-acetyl transferase (NAT) enzyme is responsible for the formation of these derivatives. The N-hydroxylation is mediated by hepatic cytochrome P450 isoforms such as CYP3A4, forming N-hydroxylated metabolites such as dapsone hydroxylamine (DDS-NHOH) and mono-acetyl dapsone hydroxylamine (MADDS-NHOH) [4]. These metabolites oxidize the Fe2+ to Fe3+ of hemoglobin forming methemoglobin. It is these latter metabolites that cause methemoglobinemia and hemolytic anemia from oxidative stress [2],[5],[6]. Dosages above 200 mg/day are usually most frequently associated with methemoglobinemia [5],[7],[8],[9][10],[11],[12].
Mechanism of methemoglobinemia
Hemoglobin is a tetrameric molecule consisting of four intertwined chains of heme. Normally, the iron stored is in ferrous (Fe2+) state which makes it competent to hold oxygen molecules. Methane level in blood is <1%, but its increase oxidizes iron from its ferrous (Fe2+) state to ferric (Fe3+) which is unyielding to carry oxygen ultimately leading to tissue hypoxia because of reduced oxygen carrying capacity of hemoglobin. The phenomenon is called methemoglobinemia. Generally, it shifts the oxygen-dissociation curve to the left side.
Methemoglobinemia could be congenital or acquired. Genetically it could be due to deficiency of the enzyme diaphorase I (cytochrome b5 reductase), methemoglobin levels rise and the blood of met-Hb patients has reduced oxygen-carrying capacity. Instead of being red in color, the arterial blood of met-Hb patients is brown. This results in the skin of white patients gaining a bluish hue. Hereditary met-Hb is caused by a recessive gene. Another reason could be presence of hemoglobin variants such as HbM and HbH. One of the major acquired cause is the exposure to exogenous oxidizing drugs and their metabolites (e.g., benzocaine, dapsone, and nitrates) may lead to an increase of up to a 1000-fold of the methemoglobin formation rate, overwhelming the protective enzyme systems and acutely increasing methemoglobin levels.
Signs and symptoms
Clinical symptoms of methemoglobinemia depend on the serum concentration of methemoglobin. Peripheral and central cyanosis are usually seen at a serum methemoglobin level of 15%. Methemoglobin levels of 30–45% result in headache, fatigue, tachycardia, weakness, and dizziness, while levels above 60% result in cardiac arrhythmia, dyspnea, seizures, and coma. Death typically occurs at methemoglobin levels greater than 70% [5].
Diagnosis
Diagnosis is one of the mainstays in methemoglobinemia. Methods to do so are varied but majorly arterial blood gas analysis is considered as a confirming test. It clearly indicates the presence and percentage of methane in Hb. Arterial blood with an elevated methemoglobin level has a characteristic chocolate-brown color as compared to normal bright red oxygen-containing arterial blood; the color can be compared with reference charts.
SpO2 concentration, as measured by pulse oximetry, is falsely high because methemoglobin absorbs the pulse oximeter light at the two wavelengths it uses to calculate the ratio of oxyhemoglobin and deoxyhemoglobin. For example, with a methemoglobin level of 30–35%, this ratio of light absorbance is 1.0, which translates into a falsely high SpO2 of 85%. Therefore, the typical oxygen “saturation gap” observed between arterial blood gas analysis and pulse oximetry readings is helpful for making the diagnosis of methemoglobinemia.
Management
The line of management is varied in different people because it depends on various factors, such as the blood methane levels, the level of G6PD enzyme, electrolyte balance, and the amount of cyanosis. Initial management is still the removal of the offending agent. If the blood methane levels are between 15% and 20% or above, the administration of methylene blue is a standardized regimen unless contraindicated. It is given intravenously 1–2 mg/kg in a drip of normal saline every hourly if the level of methane toxicity is more and through oral formulation if the toxicity is less. Methylene blue in the presence of NADP+ gets converted to leukomethylene blue and converting the former to NADPH. This leukomethylene subsequently acts as an artificial electron donor and converts the ferric (Fe3+) form of iron to ferrous (Fe2+) form which has more affinity toward oxygen thus shifting the oxygen dissociation curve from the left to its normal occurrence. Oxygen supplementation has also proved quite effective to attentuate symptoms of dyspnea. If no treatment works and symptoms persist despite the standard therapies, hemodialysis maybe required (Figure 4).
Since our patient has her G6PD enzyme level at the below normal range, methylene blue administration is contraindicated because the latter needs enzyme to work on its mechanism, if deficient would lead to oxidative stress and worsen the condition. Therefore, Vitamin C is administered intravenously with a pint. The loading dose was 10 mg/dL and was given every 6 hours. The main mechanism of Vitamin C is not well understood but is postulated that it removes oxidative stress, the main causative factor of methemoglobinemia. The methemoglobin levels showed a decrease by 1.1% with every dose instilled. Intravenous Vitamin C was given for consecutive 3 days (around 42 hours after diagnosis) intravenously with other multivitamin injections and later an oral formulation was prescribed during discharge. The start and stop times of intravenous Vitamin C therapy are mentioned in (Figure 5), along with periodic monitoring of ABGA levels.
Conclusion
Methemoglobinemia is a rare cause of hypoxia and cyanosis. It can be a potentially fatal disease if not addressed in a timely manner or left untreated. Our case emphasizes the importance of history taking, knowledge, and a high index of suspicion of drug-induced methemoglobinemia, especially in the presence of a saturation gap. Methemoglobin levels are available on modern ABG analysis and aid in appropriate and timely diagnosis. Methylene blue is the recommended treatment, with alternative treatments including high-dose Vitamin C, hyperbaric oxygen, exchange transfusions, or activated charcoal. Clinicians should be aware of the signs and symptoms of methemoglobinemia and include them in the differential diagnosis.
REFERENCES
1.
Shenouda M, Padilla M, Silva J, Castillo H, Austin A. Dapsone-induced methemoglobinemia: A case report. Cureus 2022;14(2):e22466. [CrossRef]
[Pubmed]

2.
Mansouri A, Lurie AA. Concise review: Methemoglobinemia. Am J Hematol 1993;42(1):7–12. [CrossRef]
[Pubmed]

3.
O'Dwyer D, McElvaney NG. A case of dapsone induced methaemoglobinaemia. Ir J Med Sci 2008;177(3):273–5. [CrossRef]
[Pubmed]

4.
Jollow DJ, Bradshaw TP, McMillan DC. Dapsone-induced hemolytic anemia. Drug Metab Rev 1995;27(1–2):107–24. [CrossRef]
[Pubmed]

5.
Kabir H, Lakshmanan R, Gopinath S, Bhonagiri D. Dapsone-induced methemoglobinemia—A case report. Clin Case Rep 2021;9(5):e04054. [CrossRef]
[Pubmed]

6.
Zosel A, Rychter K, Leikin JB. Dapsone-induced methemoglobinemia: Case report and literature review. Am J Ther 2007;14(6):585–7. [CrossRef]
[Pubmed]

7.
Richardson SR, O'Malley GF. Glucose-6-Phosphate Dehydrogenase Deficiency. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2025.
[Pubmed]

8.
9.
Pahal P, Goyal A. Central and Peripheral Cyanosis. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2025.
[Pubmed]

10.
Graff DM, Bosse GM, Sullivan J. Case report of methemoglobinemia in a toddler secondary to topical dapsone exposure. Pediatrics 2016;138(2):e20153186. [CrossRef]
[Pubmed]

11.
Darling RC, Roughton FJW. The effect of methemoglobin on the equilibrium between oxygen and hemoglobin. Am J Physiol 1942;137(1):56–68. [CrossRef]

12.
Park SY, Lee KW, Kang TS. High-dose vitamin C management in dapsone-induced methemoglobinemia. Am J Emerg Med 2014;32(6):684.e1–3. [CrossRef]
[Pubmed]

SUPPORTING INFORMATION
Author Contributions
Nandini Kaushik Dave - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Guarantor of SubmissionThe corresponding author is the guarantor of submission.
Source of SupportNone
Consent StatementWritten informed consent was obtained from the patient for publication of this article.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Conflict of InterestAuthor declares no conflict of interest.
Copyright© 2025 Nandini Kaushik Dave. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.