|
Case Report
Unilateral optic neuritis with contralateral central retinal artery and central retinal vein occlusion in a post-COVID-19 case of rhino-orbital-cerebral mucormycosis: An unusual presentation
1 Ophthalmology, Consultant Vitreo-Retina and Uvea, Department of Retina-Vitreous and Uvea, Amritsar Eye Clinic, Dehradun, Uttarakhand, India
2 Radiodiagnosis, Assistant Professor, Department of Radiodiagnosis and Imaging, Teerthanker Mahaveer Medical College and Research Centre Meerut, Uttar Pradesh, India
3 Radiodiagnosis, Consultant Radiodiagnosis, Department of Radio diagnosis and Imaging, Dr. Parashars Pathology and Imaging Centre, Dehradun, Uttarakhand, India
4 Medicine, Associate Professor, Department of Medicine, Shri Guru Ram Rai Institute of Medical and Health Sciences and Shri Mahant Inderesh Hospital, Dehradun, Uttarakhand, India
5 Ophthalmology, Consultant and Chief Cataract and Refractive Surgeon, Department of Cataract and Refractive Surgery, Amritsar Eye Clinic, Dehradun, Uttarakhand, India
Address correspondence to:
Deepesh Arora
122/1, Municipal Road, Dehradun, Uttarakhand,
India
Message to Corresponding Author
Article ID: 101312Z01DA2022
Access full text article on other devices

Access PDF of article on other devices

How to cite this article
Arora D, Sharma A, Raman R, Parasher A, Ahamad N, Sharma D. Unilateral optic neuritis with contralateral central retinal artery and central retinal vein occlusion in a post-COVID-19 case of rhino-orbital-cerebral mucormycosis: An unusual presentation. Int J Case Rep Images 2022;13(1):6–14.ABSTRACT
Introduction: The corona virus disease (COVID)-19 is a severe acute respiratory syndrome (SARS-CoV-2) which is posing a great threat to mankind and has been associated with a high risk of opportunistic fungi infection presenting as rhino-orbital-cerebral-mucormycosis. We report a rare and never reported case of unilateral optic neuritis with contralateral central retinal artery (CRA) and central retinal vein (CRV) occlusion in a patient of post-COVID-19 rhino-orbital-cerebral-mucormycosis.
Case Report: A 45-year-old diabetic, Indian gentleman reported to our clinic in Dehradun, Uttarakhand, India with complaints of bilateral diminution of vision in right eye (RE) five days and left eye (LE) five weeks duration. He provided recent history of COVID-19 infection for which he was hospitalized and treated. Fundus examination confirmed optic neuritis in right eye and a combined established central retinal artery and vein occlusion in left eye. Fundus fluorescein angiography, visual evoked potential, and magnetic resonance imaging (MRI) were crucial in clinching the diagnosis.
Conclusion: Rhino-orbital-cerebral-mucormycosis invades tissue through multiple routes. However, it is extremely rare to see a combination of angioinvasion leading to visual loss in one eye and perineural spread leading to optic neuritis and visual loss in the contralateral eye. Clinicians must be aware of such rare presentations which could serve as benchmark in diagnosis and treatment.
Keywords: Angioinvasion, Mucormycosis, Optic neuritis, Perineural invasion
Introduction
The corona virus disease (COVID)-19 is a severe acute respiratory syndrome (SARS-CoV-2) which is posing a great threat to mankind and its health, primarily presents as respiratory disease [1]. High risk of opportunistic fungal infections (33%), namely aspergillosis and mucormycosis, has been seen with SARS-CoV-2 infection more so in severely ill SARS patients [2]. Certain conditions predisposing to fungal infections are diabetes mellitus, use of corticosteroids, immunodeficiency, immunosuppression, malignancies, patients on cell/tissue/organ transplant, and patients on ventilator support [3],[4]. Mucormycosis which is caused by fungus mucor (Class Phycomycetes, order Mucorales) has been closely associated with SARS-CoV-2 pandemic and frequently invades craniofacial compartments, pharynx, orbit, paranasal sinuses (PNS) and intracranial cavity via spores, invasion of bones, perineural spread, and angioinvasion [5], to involve intracranial vasculature and deep seated cavernous sinuses [6]. There has been high propensity of cases of SARS-CoV-2 infection and mucormycosis reported from India in the second wave of COVID-19.
We report a rare first of its kind case of unilateral optic neuritis with contralateral central retinal artery and central retinal vein occlusion in a patient with rhino-orbital-cerebral-mucormycosis.
Case Report
A 45-year-old Indian gentleman, with well controlled type II diabetes mellitus (HgbA1c 6.4%) consulted on June 5, 2021 at Amritsar Eye Clinic, Dehradun, Uttarakhand, India for a 5-day history of sudden onset of painless visual loss in right eye with poor navigational vision and painful extraocular movements with facial asymmetry, right sided facial swelling, and blackish stained nasal discharge. He also complained of poor vision in the left eye of five weeks duration presenting with left eye swelling and inability to move the eye ball.
Five weeks prior he provided history of corona virus infection (COVID-19) which was confirmed by real time-polymerase chain reaction (RT-PCR) test from nasopharyngeal swab for SARS-CoV-2 RNA which was positive. His detailed hospital records were informatory and showed that he had become symptomatic following chest infection presenting as breathlessness and cough around the 8th day of fever needing hospital admission. Records revealed computed axial tomography (CAT)-scan of chest with bilateral ground glass appearance with progressive consolidation placing it at peak stage. Clinical examination had detected low oxygen saturation by pulse oximetry (SpO2) at 88% while breathing ambient air needing urgent intensive care unit (ICU) admission. There was history of treatment with steroids, antivirals, and immunomodulators (Dexamethasone in combination with Remdesivir as per COVID-19 guidelines), remdesivir (Remdesivir (GS-5734), an inhibitor of the viral RNA-dependent, RNA polymerase, administered as intravenous (IV) 200 mg on day one, followed by 100 mg IV for 9 days), and tocilizumab (humanized anti-IL-6 receptor antibody administered as 4–8 mg/kg body weight administered as single 60-minute intravenous infusion repeated at four weeks). During his hospital stay he was diagnosed with left side stage 3b rhino-orbital-cerebral-mucormycosis (ROCM). Primary initiation of medical management with intravenous liposomal amphotericin B (AMB) 1.0 mg/kg/body weight was given for 22 days, retrobulbar injection of amphotericin B deoxycholate was given in five doses (3.5 mg of AMB deoxycholate preceded by retrobulbar injection of 2 mL of 2% lidocaine (anesthetic). The patient underwent left sided primary functional endoscopic sinus surgery (FESS) which included maxillary antrostomy, ethmoidectomy, and sphenoidectomy. Biopsy was taken and it showed septate hyphae confirming mucormycosis. At this stage he had developed left sided ptosis, proptosis, total ophthalmoplegia with fixed mid-dilated pupil.
During his ongoing treatment he developed left sided cavernous sinus thrombosis, frontal lobe infarct, and right sided hemiparesis. There was no personal or family history related to neurological disease or immune pathology.
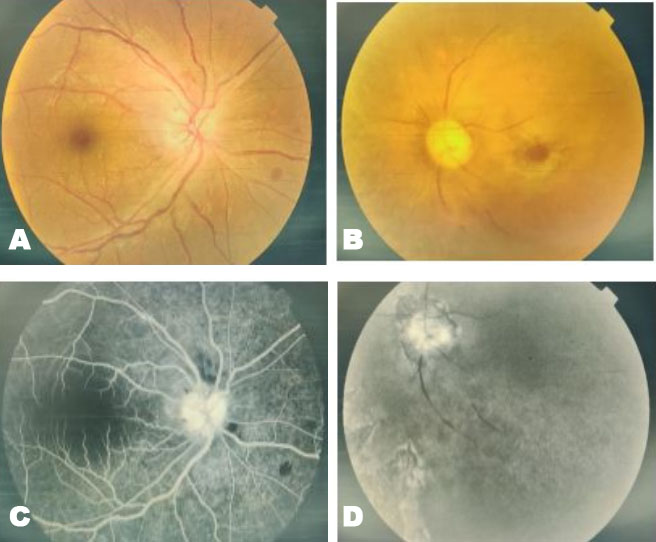
Physical examination of right side of face showed black nasal discharge encrusted in anterior part of right nostril. On ocular examination, the right eye visual acuity (Va) was 6/36 and left eye (Va) was hand movement (HM). Right eye color and contrast perception was inaccurate. Anterior segment examination of right eye detected grade 2 relative afferent pupillary defect (RAPD) with grade 1+cells and flare. Right eye posterior segment showed vitreous haze, vitritis (grade 1), optic disc edema with splinter hemorrhage involving superior pole (Figure 1A). Left eye showed proptosis, ptosis, periorbital swelling, conjunctival chemosis, and fixed mid dilated pupil with total ophthalmoplegia. Fundus showed optic disc pallor, central retinal artery with central retinal vein occlusion and presence of diffuse blackish pigmentation seen extending from posterior pole to involve retinal periphery (Figure 1B). The patient underwent immediate admission at a tertiary care hospital in Dehradun and thereafter further multidisciplinary treatment was planned as per the advice of medical specialist and team.
Fundus fluorescein angiography (FFA) showed right eye disc leakage with normal filling of arterial and venous tree (Figure 1C). Left eye showed no vascular filling with near total loss of vascular architecture. It showed irregular disc margins with loss of retinal pigment epithelium (RPE). Isolated areas of pigmentation with RPE loss was seen in periphery (Figure 1D). Spectral domain optical coherence tomography (SD-OCT) (OPTOVUE) showed normal retina map and retinal nerve fiber layer in right eye (Figure 2A). Automated HVF 30-2 (Figure 2B) and HVF 10-2 visual fields (Figure 2C) (HFA II, Humphrey Field Analyzer II) (Carl Zeiss Meditec Inc, Dublin, CA, 2007) showed severely depressed visual field sparing a small sector corresponding to inferotemporal quadrant in right eye. Magnetic resonance imaging (MRI) of brain detected frontal lobe infarct. Magnetic resonance imaging axial view (Figure 3A) revealed retrobulbar intra-orbital segment of the bilateral optic nerve which appeared mildly bulky with a high T2 signal in right optic nerve which was suggestive of bilateral optic neuritis. Magnetic resonance imaging axial view (Figure 3B) revealed poorly defined mucosal thickening involving bilateral maxillary sinus appearing hyperintense on T2 sequence with few internal areas of T2 hypointensity depicting fungal element with absence of medial wall of left maxillary sinus and floor of left orbit. Magnetic resonance imaging coronal view (Figure 3C) revealed soft tissue extension to bilateral orbits with edema and fat stranding involving bilateral orbital fat suggestive of bilateral orbital cellulitis. Visual evoked potential (VEP) showed flattened wave on left side and markedly delayed P100 latency on right side indicative of residual optic nerve damage.
Blood picture revealed elevated erythrocyte sedimentation rate (ESR 46 mmHg) (Range: men over 50 years: 0–20 mm/h). Total blood picture, renal and hepatic enzyme panel, serum electrolytes were in range. Lumbar puncture was done with findings of colorless cerebrospinal fluid (CSF), red blood cell count of 0 cells/mm3 (Range: <1 RBC/mm3), white blood cell (WBC) count of 11 cells/mm3 (Range: 0–8 leukocytes/mm3), lymphocytosis (>70%) (Normal WBC count in CSF is <5 cells/ul) [differential leukocyte count (DLC) (Normal): mononuclear cells predominate: 60–70% were small lymphocytes and 30–40% monocytes], albumin 32.4 mg/dL (Range: 8–42 mg/dL), glucose 55 mg/dL (Range: 40–70 mg/dL), and total protein 40 mg/dL (Range: 15–60 mg/dL). Culture and sensitivity was sterile. Oligoclonal bands were absent. CSF-SARS-CoV-2 ribonucleic acid (RNA) polymerase chain reaction (PCR) was not done. We did serology for TB gold quantiferon test, AQP4 autoantibody, Myelin oligodendrocyte glycoprotein (MOG) autoantibody, antinuclear antibody (ANA) profile full, antineutrophil cytoplasmic antibodies (ANCA) for c and p antibody, and anti-cyclic citrullinated peptides (ACCP) which were non-reactive with normal levels.
The patient was diagnosed with right eye post-COVID-19 related stage 2c rhino-orbital-mucormycosis (ROM) infiltration leading to development of optic neuritis. Topically he was started on intensive steroids 1 hourly (Predforte) with mydriatic and cycloplegic (atropine 1%) 3 times/day. He was treated on lines of optic neuritis and was given 3 consecutive doses of intravenous (IV) 1 gram of methyl prednisolone in 200 mL of 20% dextrose normal saline over 2 hours. Oral steroids were withheld taking in view presence of mucormycosis. He was started on intensive IV liposomal amphotericin B 1.0 mg/kg/bodyweight with 200 mg of posaconazole in four divided doses. Retrobulbar injection of amphotericin B deoxycholate was given in two doses (3.5 mg of AMB deoxycholate preceded by retrobulbar injection of 2 mL of 2% lidocaine (anesthetic) at a gap of three days. Strict watch was kept on serum electrolytes (potassium and magnesium), renal function (serum creatinine). Swab taken from deep nasal cavity under potassium hydroxide (KOH) fixation showed septate hyphae similar to lesion detected on left side with ROCM. Nasal cavity showed a necrotic patch near inferior turbinate from which tissue biopsy was taken and sent for histopathology. He underwent early surgical intervention with primary functional endoscopic sinus surgery (FESS) involving maxillary antrostomy and spheno-ethmoidectomy. The necrotic tissue was aggressively debrided and infected tissue was resected. Over the next eight weeks the patient started showing slow but progressive recovery. He was weaned off ventilator support system. Mucormycosis growth had been contained with borderline visual improvement. At three months he was maintaining follow-up on outpatient basis. His last recorded Va was 6/12p in RE with stable systemic parameters and moderately well controlled diabetes.
Discussion
As COVID-19 pandemic evolves there has been rapid increase in cases of mucormycosis more so of ROCM. Presentation have been variable from being asymptomatic to severe loss of vision resulting in death in some cases. Bilateral visual loss has been related to orbital presence of mucormycosis or to neurophthalmic complications.
We present a case of right eye with acute optic neuritis with maxillary sinus mucormycosis and left eye with total ophthalmoplegia with central retinal artery and central retinal vein occlusion with cavernous sinus thrombosis in a left sided post-COVID-19 ROCM.
We describe the risk factors, presentation, and probable course taken by mucormycosis in the development of these lesions. We also present case specific references related to arterial with venous occlusion and optic neuritis in context of mucormycosis.
Mucormycosis , an invasive aggressive fungus called as the deadly black fungus, is a life-threatening fungal infection belonging to order Mucorale [5],[7]. These fungi are typically found in hematological malignancies, hematopoietic stem cell transplantation, and solid organ transplantation [8], diabetics, patients with history of corticosteroids use, those on ventilator support, and patients with history of prolonged intensive care admission [3],[4]. Mucormycosis is transmitted via inhalation of spores, intake of contaminated food, and fungal inoculation into skin cuts or abrasions [8],[9]. Among various forms of mucormycosis, ROCM is most prevalent and commonly seen in diabetics [8]. The reason for mucormycosis being prevalent in diabetics is due to the fact that innate immunity in these patients interferes and impacts the phagocytosis of fungi by polyomorphonuclear leukocytes [10]. Existing COVID-19 data has shown Indian contribution toward ROCM cases stands at 81% [11], largest in the world and primarily seen in second wave of COVID-19. Rhino-orbital-cerebral-mucormycosis has been found to occur in post-recovery phase of COVID-19 or at times simultaneously. The median time for diagnosis of mucormycosis is estimated at ten days from day of COVID-19 detection [12].
Presentation of mucormycosis in the eye may be variable. The eye may be quiet appearing white, patient may present with visual loss, orbital or facial pain, nasal discharge. Examination may reveal cranial nerve palsies, proptosis, ptosis, indicating the spread of mucormycosis to involve orbital apex indicating a stronger risk of involving intracranial sinus namely cavernous and sagittal sinus. “Sen et al.” in a large study from COVID-19 pandemic involving 2826 patients with rhino-orbital-cerebral mucormycosis found the right and left paranasal sinus (PNS), orbit, and brain were equally involved with PNS involvement seen in 40% of cases [13]. Involvement of central nervous system (CNS) has been found to be 37% of COVID-19 associated ROCM [12]. “Sen et al.” have reported 21% CNS involvement with focal or diffuse cavernous sinus involvement or thrombosis seen in 53% of their cases [13].
Mucormycosis initially affects the nasal or maxillary sinuses spreading rapidly to involve ethmoidal sinus. It may enter the orbit via various routes of ethmoidal foramina, nasolacrimal duct, or by splitting lamina papyracea [14] reaching orbital apex leading to visual compromise by involving optic nerve. As mucormycosis spreads to involve the orbital apex and its various structures, it produces distinctive symptoms and signs of vision loss related to optic nerve. It can initiate ophthalmoplegia due to involvement of cranial nerves III, IV, and VI along with involvement of first division of trigeminal nerve (V1). Findings of cavernous sinus syndrome and superior orbital fissure syndrome may overlap with orbital apex syndrome due to anatomical closeness [15]. Mucormycosis is characterized by extensive angioinvasion causing vessel thrombosis and necrosis of surrounding tissue leading to infarction. It invades and involves mainly intermediate to large size arteries or venous channels. Typically fungal hyphae grow in internal elastic lamina invading the vascular lumen inhibiting vascular thrombosis. Once the vessel is involved the fungus is disseminated to distant sites causing hemorrhagic infarcts [16]. Angioinvasion occurs through cavernous sinus and internal carotid artery leading to involvement of brain parenchyma, development of infarct, mycotic abscess and aneurysm. There may be involvement of ophthalmic artery presenting as motor and sensory disorders leading to visual loss and multiple cranial nerve palsy [14].
Besides vascular invasion, mucormycosis can spread via perineural route directly through cribriform plate into the anterior cranial fossa [17]. “Sravani et al.” have demonstrated perineural spread (PS) in a study of patients affected by ROCM [16]. “Maclean et al.” were able to prove histologically PS of disease from cavernous sinus to pons along trigeminal nerve with no involvement of brain parenchyma [18]. In another case reported by “Margo et al.” V1 division of trigeminal nerve involvement was seen in anterior orbit due to extensive invasive mucormycosis [19]. “Frater et al.” who had reported high percentage of perineural invasion concluded PS was another possible mechanism of fungal extension into CNS [20]. We must remember that contrast-enhanced MRI studies have documented the presence of perineural invasion via trigeminal nerve thus confirming the route [18],[19].
Significance of knowing about PS is that it is marker of poor survival outcome and an indicator of local and regional recurrence rate [16]. Malignancies frequently seen in the neck, such as adenoid cystic carcinoma, squamous cell carcinoma, and lymphoma, lymphoma and rhabdomyosarcoma frequently spread in perineural fashion, commonly involving trigeminal nerve and its branches [21] as documented by “Maclean et al.” [18] and “Orgus et al.” [22]. “Brown et al.” are of the view that perineural sheath is a compartment located between nerve axons supporting epineural fibrous sheath. Complex interplay of trophic factors play a role in invasion of this space by tumor cells, and the process is called “neurotrophism.” The perineural sheath seems to provide a conduit for tumor spread beyond the primary site of origin. Perineural tumor growth has been found to be of two types, “perineural invasion” involving small unnamed nerves and “perineural spread” involving large named nerves. Neural sheath may behave as a low resistance conduit for growth of tumor cells. It is possible that the role of signaling among tumors’ stromal cells via paracrine mechanisms seems to be at play here. In what may bring about the early final changes in the cascade of neural spread is the upregulation of neurotrophic and axonal guidance molecules with activation of cancer cells chemotaxis. Final changes that lead to perineural spread are contributed by role of directional cell migration toward and along the nerves [23],[24].
Most frequent presentation of ROCM is ophthalmoplegia either with or without vascular occlusion. These lesions because of their location frequently lead to orbital apex syndrome presenting also as cavernous sinus thrombosis which occurs due to mucor-related angioinvasion causing vascular thrombosis. There have been classical reports on development of central retinal artery occlusion (CRAO) due to mucormycosis seen in the second wave of COVID-19. “Patnaik et al.” described an unusual case of a 66-year-old diabetic lady diagnosed with bilateral CRAO with bilateral total ophthalmoplegia and bilateral ptosis in a post-COVID-19 ROCM patient where angioinvasion may have been a factor [25]. “Bwankar et al.” have described a case of a 55-year-old gentleman diagnosed with unilateral left ptosis, total ophthalmoplegia, CRAO, multiple cranial nerve palsies (III, IV, V1, and VI) confirmed on MRI with orbital apex, superior orbital fissure syndrome eventually resulting in cavernous sinus thrombosis [26]. In a study of 2826 COVID-19 patients with ROCM, “Sen et al.” have shed greater light on the role and development of complications related to mucormycosis. In this large series they also commented that remdesivir and tocilizumab may not have played a role in increasing the risk of ROCM [13].
In fungus related sinusitis, fungi spread from PNS to involve orbital apex leading to orbital apex syndrome with multi-cranial nerve palsies and optic neuropathy. Optic neuritis constitutes one of the variable presentations of ROCM. “Sano et al.” reported a 94-year-old lady with unilateral right sided blindness due to sphenoidal sinus mucormycosis diagnosed as retrobulbar optic neuropathy with no change in visual outcome post treatment [27]. “Chen et al.” reported a 64-year-old lady of Asian descent three days post-chemotherapy with cisplatin developed visual decline, and she was confirmed on MRI with left perineuritis and maxillary sinus mucormycosis [28].
Angioinvasive spread is rapid, involving multiple tissue sites at the same time having a necrotic element to it resulting in extensive tissue damage, the clinical picture seen in our case involving left PNS, orbit, and brain parenchyma. Clinical history and imaging (MRI) indicates a rapid spread of mucormycosis from maxillary and ethmoidal sinuses involving orbital apex with invasion of cavernous sinus resulting in its thrombosis [28]. The symptom complex seen occurred due to structural involvement of optic nerve, multiple cranial nerves (III, IV, branches V1 and V2 of V nerve, and VI nerve), superior and inferior ophthalmic vein and its tributaries, the internal carotid artery and brain parenchyma, manifesting as CRA with CRV occlusion and frontal lobe infarct.
Magnetic resonance imaging and VEP have provided much needed support in tracing spread of mucormycosis from maxillary sinus to the optic nerve and confirming the presence of optic neuritis. There have been various references in relation to PS [16],[17] in ROCM patients [16]. “Margo et al.” have also documented spread via V1 division of trigeminal nerve [19]. We believe [20] that the spread to optic nerve occurred either via a small or large nerve [23] which could act as conduit for spread of mucormycosis to eye and CNS. The role of vascular trauma to the nerve fiber layer (optic nerve) seems to have been minimal indicated by the visual recovery in the patient. Resolution of tissue and fat inflammation with edema could also be a contributory factor in visual gain. We must remember that there is a complex interplay of multiple factors that would be finally responsible in predicting outcome.
Conclusion
Mucormycosis is a disease with multiple presentations and its manifestations may be atypical. It is imperative to remember that there may be more than one route for transmission of fungi leading to simultaneous presentation of two separate disease entities with a very short time interval between them. Clinicians must be aware of this as more waves of COVID-19 of different intensity are expected in near future.
REFERENCES
1.
Fisher D, Heymann D. Q&A: The novel coronavirus outbreak causing COVID-19. BMC Med 2020;18(1):57. [CrossRef]
[Pubmed]

2.
Song G, Liang G, Liu W. Fungal co-infections associated with global COVID-19 pandemic: A clinical and diagnostic perspective from China. Mycopathologia 2020;185(4):599–606. [CrossRef]
[Pubmed]

3.
Honavar SG. Code mucor: Guidelines for the diagnosis, staging and management of rhino-orbito-cerebral mucormycosis in the setting of COVID-19. Indian J Ophthalmol 2021;69(6):1361–5. [CrossRef]
[Pubmed]

4.
Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir Med 2020;8(5):475–81. [CrossRef]
[Pubmed]

5.
Ravani SA, Agrawal GA, Leuva PA, Modi PH, Amin KD. Rise of the phoenix: Mucormycosis in COVID-19 times. Indian J Ophthalmol 2021;69(6):1563–8. [CrossRef]
[Pubmed]

6.
Morace G, Borghi E. Invasive mold infections: Virulence and pathogenesis of mucorales. Int J Microbiol 2012;2012:349278. [CrossRef]
[Pubmed]

7.
Chegini Z, Didehdar M, Khoshbayan A, Rajaeih S, Salehi M, Shariati A. Epidemiology, clinical features, diagnosis and treatment of cerebral mucormycosis in diabetic patients: A systematic review of case reports and case series. Mycoses 2020;63(12):1264–82. [CrossRef]
[Pubmed]

8.
Jeong W, Keighley C, Wolfe R, et al. The epidemiology and clinical manifestations of mucormycosis: A systematic review and meta-analysis of case reports. Clin Microbiol Infect 2019;25(1):26–34. [CrossRef]
[Pubmed]

9.
Prakash H, Chakrabarti A. Global epidemiology of mucormycosis. J Fungi (Basel) 2019;5(1):26. [CrossRef]
[Pubmed]

10.
Rammaert B, Lanternier F, Zahar JR, et al. Healthcare-associated mucormycosis. Clin Infect Dis 2012;54 Suppl 1:S44–54. [CrossRef]
[Pubmed]

11.
Singh AK, Singh R, Joshi SR, Misra A. Mucormycosis in COVID-19: A systematic review of cases reported worldwide and in India. Diabetes Metab Syndr 2021;15(4):102146. [CrossRef]
[Pubmed]

12.
Hoenigl M, Seidel D, Carvalho A, et al. The emergence of COVID-19 associated mucormycosis: Analysis of cases from 18 countries. 2021. [Available at: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3844587]

13.
Sen M, Honavar SG, Bansal R, et al. Epidemiology, clinical profile, management, and outcome of COVID-19-associated rhino-orbital-cerebral mucormycosis in 2826 patients in India – Collaborative OPAI-IJO Study on Mucormycosis in COVID-19 (COSMIC), Report 1. Indian J Ophthalmol 2021;69(7):1670–92. [CrossRef]

14.
Mahalaxmi I, Jayaramayya K, Venkatesan D, et al. Mucormycosis: An opportunistic pathogen during COVID-19. Environ Res 2021;201:111643. [CrossRef]
[Pubmed]

15.
Badakere A, Patil-Chhablani P. Orbital apex syndrome: A review. Eye Brain 2019;11:63–72. [CrossRef]
[Pubmed]

16.
Sravani T, Uppin SG, Uppin MS, Sundaram C. Rhinocerebral mucormycosis: Pathology revisited with emphasis on perineural spread. Neurol India 2014;62(4):383–6. [CrossRef]
[Pubmed]

17.
18.
McLean FM, Ginsberg LE, Stanton CA. Perineural spread of rhinocerebral mucormycosis. AJNR Am J Neuroradiol 1996;17(1):114–6.
[Pubmed]

19.
Margo CE, Linden C, Strickland-Marmol LB, Denietolis AL, McCaffrey JC, Krik N. Rhinocerebral mucormycosis with perineural spread. Ophthalmic Plast Reconstr Surg 2007;23(4):326–7 [CrossRef]
[Pubmed]

20.
Frater JL, Hall GS, Procop GW. Histologic features of zygomycosis: Emphasis on perineural invasion and fungal morphology. Arch Pathol Lab Med 2001;125(3):375–8. [CrossRef]
[Pubmed]

21.
Parker GD, Harnsberger HR. Clinical-radiologic issues in perineural tumor spread of malignant diseases of the extracranial head and neck. Radiographics 1991;11(3):383–99. [CrossRef]
[Pubmed]

22.
Orguc S, Yücetürk AV, Demir MA, Goktan C. Rhinocerebral mucormycosis: Perineural spread via the trigeminal nerve. J Clin Neurosci 2005;12(4):484–6. [CrossRef]
[Pubmed]

23.
Brown IS. Pathology of perineural spread. J Neurol Surg B Skull Base 2016;77(2):124–30. [CrossRef]
[Pubmed]

24.
Bakst RL, Lee N, He S, et al. Radiation impairs perineural invasion by modulating the nerve microenvironment. PLoS One 2012;7(6):e39925. [CrossRef]
[Pubmed]

25.
Patnaik A, Sharma B, Ahmad R, Kumar A, Chitrotpala R, Gupta M. A case of bilateral central retinal artery occlusion in a post-COVID rhino-orbital-cerebral mucormycosis patient. Cureus 2021;13(11):e20062. [CrossRef]
[Pubmed]

26.
Bawankar P, Lahane S, Pathak P, Gonde P, Singh A. Central retinal artery occlusion as the presenting manifestation of invasive rhino-orbital-cerebral mucormycosis. Taiwan J Ophthalmol 2020;10(1):62–5. [CrossRef]
[Pubmed]

27.
Sano T, Kobayashi Z, Takaoka K, et al. Retrobulbar optic neuropathy associated with sphenoid sinus mucormycosis. Neurol Clin Neurosci 2018;6(5):146–7. [CrossRef]
[Pubmed]

28.
Chen IW, Lin CW. Rhino-orbital-cerebral mucormycosis. CMAJ 2019;191(16):E450. [CrossRef]
[Pubmed]

SUPPORTING INFORMATION
Author Contributions
Deepesh Arora - Conception of the work, Design of the work, Acquisition of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Anuj Sharma - Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ratish Raman - Analysis of data, Drafting the work, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ankit Parasher - Acquisition of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Niyaaz Ahamad - Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Dinesh Sharma - Drafting the work, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Guarantor of SubmissionThe corresponding author is the guarantor of submission.
Source of SupportNone
Consent StatementWritten informed consent was obtained from the patient for publication of this article.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Conflict of InterestAuthors declare no conflict of interest.
Copyright© 2022 Deepesh Arora et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.