|
Case Report
Eosinophilic granulomatosis with polyangiitis presenting with acute polyneuropathy resembling Guillain–Barre syndrome
1 Department of Neurology, Mohamed V Military Teaching Hospital, Mohamed V University, Rabat, Morocco
2 Department of Neurophysiology, Mohamed V Military Teaching Hospital, Mohamed V University, Rabat, Morocco
Address correspondence to:
Mohamed Hamid
Department of Neurology, Mohamed V Military Teaching Hospital, Mohamed V University, Rabat,
Morocco
Message to Corresponding Author
Article ID: 101282Z01MH2022
Access full text article on other devices

Access PDF of article on other devices

How to cite this article
Hamid M, Benmoh Y, Moussavou C, Ahizone A, Bakal A, Ajamate M, Satte A, Bourazza A. Eosinophilic granulomatosis with polyangiitis presenting with acute polyneuropathy resembling Guillain–Barre syndrome. Int J Case Rep Images 2022;13:101282Z01MH2022.ABSTRACT
Introduction: Eosinophilic granulomatosis with polyangiitis (EGPA) is defined as a small- and medium-sized artery necrotizing vasculitis associated with asthma, eosinophilia, and extra neurologic granulomatosis (lung, cardiac, kidney, and skin). We report a case of EGPA with Guillain–Barre syndrome (GBS) like presentation.
Case Report: A 37-year-old man with a history of asthma was admitted for rapidly progressive symmetric flaccid areflexic tetraparesis, peripheral facial palsy, proprioceptive hypoesthesia, and thigh skin purpuric lesions. Electroneuromyography study revealed demyelinating sensory motor polyneuropathy with secondary axonal loss. Cervical MRI, cerebrospinal fluid study, and paraclinical tests were normal. Complete blood count showed hypereosinophilia and elevated erythrocyte sedimentation. Electrocardiogram and transthoracic echocardiography were normal. Spirometry revealed obstructive syndrome. Chest and paranasal sinus computed tomography (CT) demonstrated ground-glass opacities and severe pansinusitis. Skin biopsy showed necrotizing vasculitis with eosinophils and antineutrophil cytoplasmic antibody (ANCA) was negative. Clinical, laboratory, and radiologic findings met the American College of Rheumatology (ACR) EGPA criteria. The patient was treated by methylprednisolone bolus and cyclophosphamide with sensory motor recovery and no systemic relapse with 10 months follow-up.
Conclusion: Eosinophilic granulomatosis with polyangiitis may be revealed by a GBS mimicking presentation. Neurologic system can be involved in the ANCA negative EGPA. Paraclinical tests should be performed in GBS presentation to make accurate diagnosis and early treatment.
Keywords: Asthma, Eosinophilic granulomatosis with polyangiitis, Guillain–Barre syndrome, Hypereosinophilia
Introduction
First described in 1951, Churg–Strauss syndrome is a systemic necrotizing vasculitis affecting small and medium vessels, associated with asthma and eosinophilia [1]. In 40%, the now named Eosinophilic granulomatosis with polyangiitis (EGPA) is associated with antineutrophil cytoplasmic antibodies (ANCA). Eosinophilic granulomatosis with polyangiitis causes multisystemic disorders, involving lung, cardiac, skin lesions, and peripheral neuropathy [2]. We report a case of EGPA with Guillain–Barre syndrome (GBS) like presentation.
Case Report
Previously treated for asthma, a 37-year-old man was admitted for rapidly progressive lower limb weakness. He had no history of diabetes, tuberculosis, smoking, or alcoholism. Three weeks before his admission, the patient was treated for tracheobronchitis. Two weeks later, he reported progressive paresthesia and numbness in both lower limbs. Three days later neurologic symptoms advanced to both upper limbs. The evolution was marked by ascending symmetric paralysis with neuropathic pain. Clinical examination found conscious patient, with supple neck. Neurological examination revealed symmetric flaccid areflexic proximodistal tetraparesis quoted to 1/5 on Medical Research Council (MRC) grading scale; proprioceptive hypoesthesia and peripheral facial palsy. Apart from thigh skin purpuric lesions, there were no other extra-neurological signs.
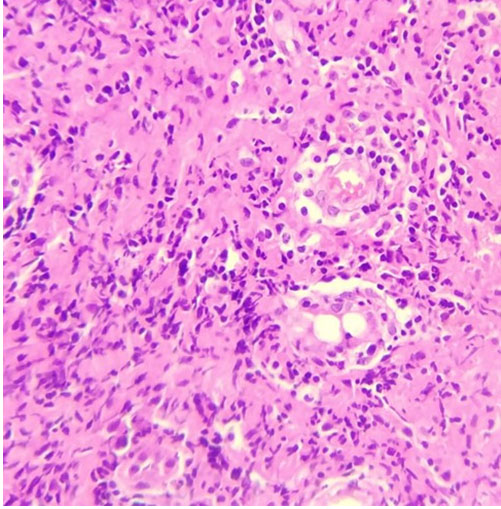
Guillain–Barre syndrome was suspected. Electroneuromyography demonstrated conduction block, prolonged distal motor latency with slowing motor nerve velocity and F wave absence in peroneal and tibial nerves. Sural nerve sensitive response amplitude was decreased. We conclude to demyelinating sensory motor polyneuropathy with secondary axonal loss (Table 1). Cervical MRI showed no signal abnormality and no cervical roots gadolinium enhancement. There was no albuminocytologic dissociation on cerebrospinal fluid study. Paraclinical tests were normal (thyroid hormone, B12, and B9 seric levels, serology for hepatitis B, C, HIV, syphilis, tuberculosis polymerase chain reaction (PCR), salivary gland biopsy, renal and hepatic tests).
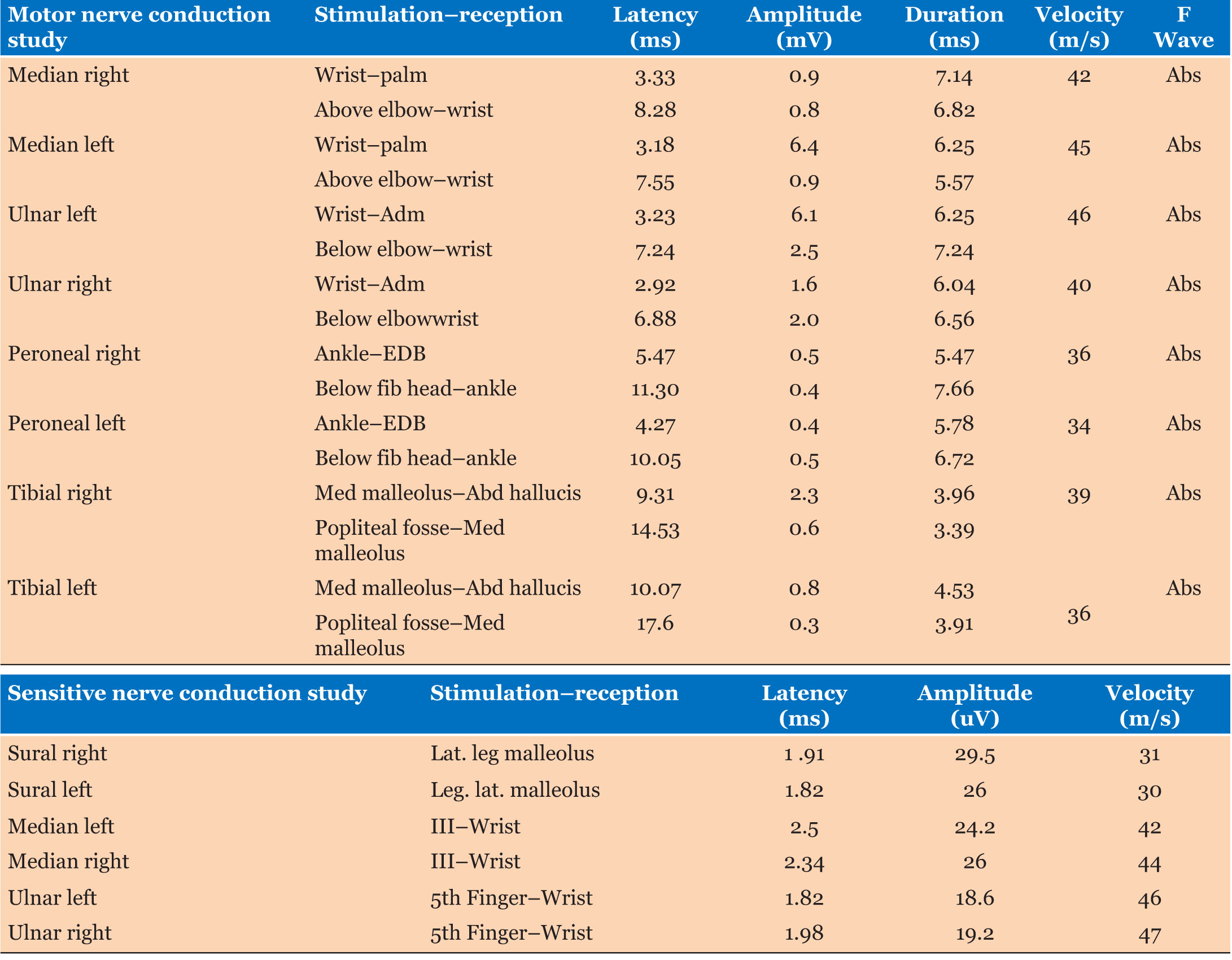
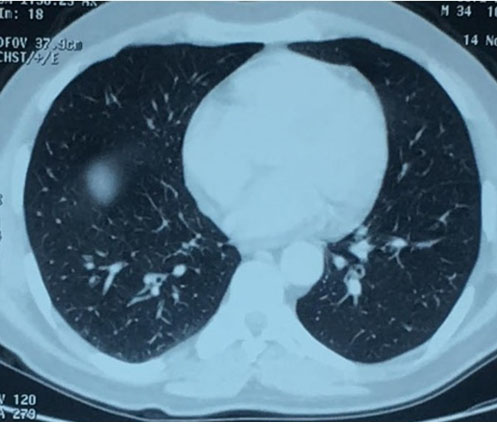
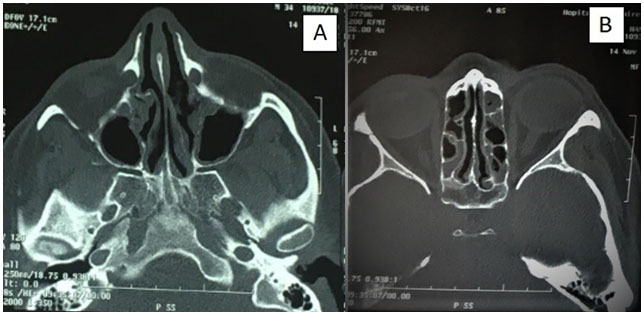
Complete blood count (CBC) showed hypereosinophilia to 7200 cells/mm3 with elevated erythrocyte sedimentation rate to 49 mm/h. Spirometry revealed obstructive syndrome. Electrocardiogram and transthoracic echocardiography were normal. Chest and paranasal sinus CT demonstrated ground-glass opacities (Figure 1) and severe pansinusitis (Figure 2). Skin biopsy showed necrotizing vasculitis with eosinophils (Figure 3) and ANCA were negative.
Eosinophilic granulomatosis with polyangiitis was evoked on clinical, electrophysiological, and radiological findings. Histological results confirmed the diagnosis.
The patient was treated with methylprednisolone bolus (1 g/day for three days) followed by cyclophosphamide 600 mg/m2 at day 1(D1), D15, D30 then monthly. He also underwent functional rehabilitation. Evolution was favorable, with sensory and motor gradual recovery and no systemic relapse with 10 months follow-up.
Conduction block in right tibial nerve, left ulnar nerve, left median nerve with decreased motor amplitude response with slowing motor conduction velocity in right median, right ulnar, and both peroneal nerves. Absence of F wave of all nerves. Slowing sensitive conduction velocity with normal sensitive amplitude response in both sural nerves. Conclusion: demyelinating motor-sensory polyneuropathy with secondary axonal loss.
Discussion
Eosinophilic granulomatosis with polyangiitis is defined as a small- and medium-sized arteries necrotizing vasculitis associated with asthma, eosinophilia, and extra-neurologic granulomatosis. Its annual incidence is above 0.5 to 4.2 per million people, more frequent in people aged between 40- and 60-year-old with no gender predominance or ethnic predisposition [3],[4],[5].Eosinophilic granulomatosis with polyangiitis evolves through three phases: prodromic phase marked by recurrent sinusitis, nasal polyposis, and bronchial asthma; eosinophilic phase marked by eosinophilia and organ involvement (lung, cardiac, skin, kidney) and generalized or vasculitic stage with small vessel involvement [6].
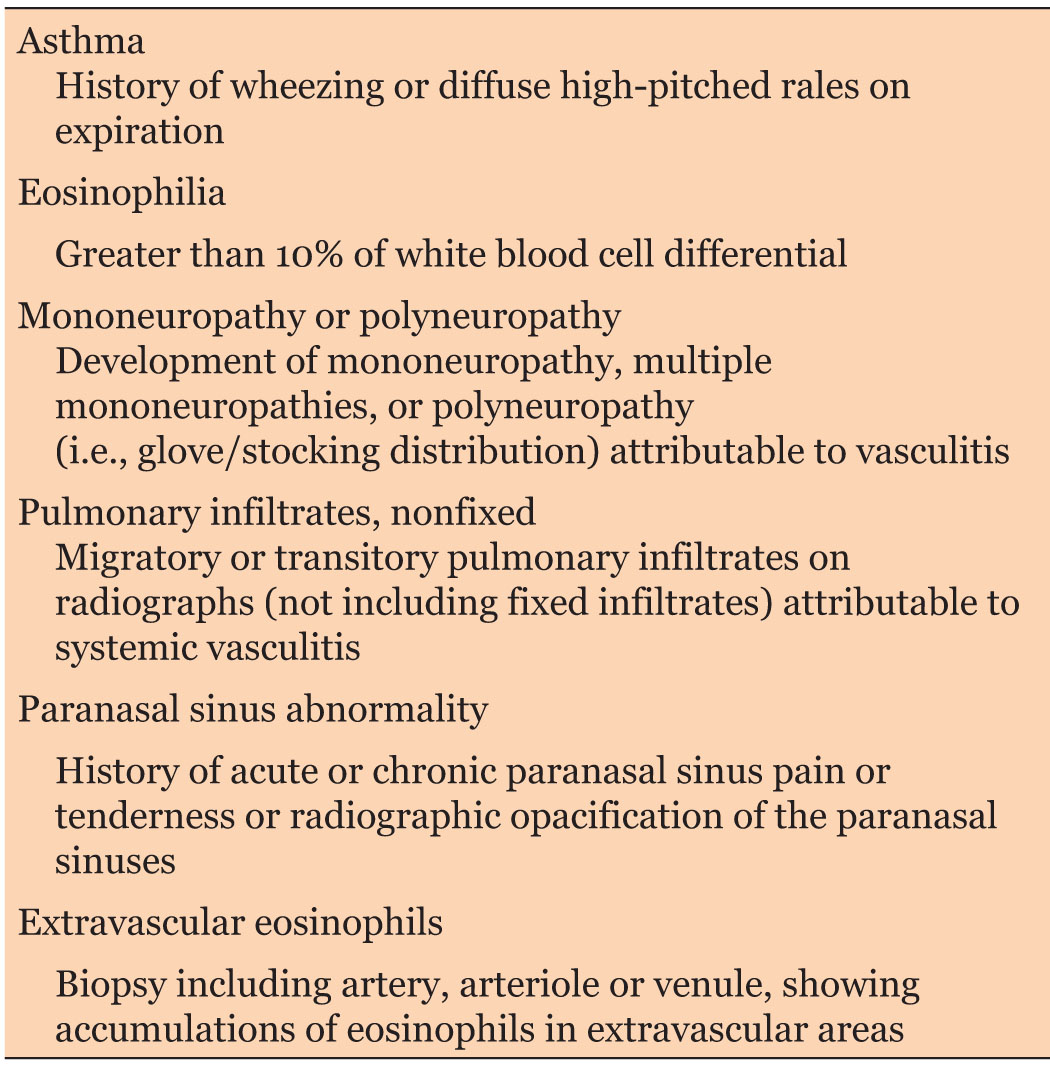
Diagnostic of EGPA is established by American College of Rheumatology (ACR) and made when four of the six criteria are met (Table 2) [1]. Case reported met the ACR criteria: acute demyelinating polyneuropathy, asthma, eosinophilia, severe pansinusitis, and necrotizing vasculitis with eosinophils on skin biopsy.
Peripheral nervous system involvement is reported in up to 76%. The typical form is multiple mononeuropathies followed by polyneuropathy (24%) and less frequently lumbar radiculopathy (3%). Central nervous system involvement is less common (seizure, confusion, and coma) but more severe regarding morbidity and mortality among patient with EGPA [7]. Ischemic optic neuropathy is the most common cranial nerve lesion reported. Facial nerve palsy as mentioned in our case is rarely reported over the literature [8]. Eosinophilic granulomatosis with polyangiitis can be differentiate in two subsets, ANCA-positive subset occurred in 38% of EGPA patients frequently presented glomerulonephritis and peripheral neuropathy, and ANCA-negative subset with lung infiltrates and endo-myocardial involvement [9]. Increased peripheral or central nervous involvement among patient with ANCA positive are frequently reported, but it doesn’t in our case. Histopathological findings of vasculitic neuropathy are marked by axonal degeneration of nerve fibers, vasculitis ischemia which induced by eosinophils derived neurotoxins. Nerve biopsy is no more mandatory criteria for EGPA diagnosis [10],[11]. In the Sable-Fourtassou et al. study [12], only 39% of the 69 EGPA patients with ANCA-negative had features of vasculitis on biopsy. Our patient had ANCA-negative with features of eosinophilia on skin biopsy. Eosinophilic granulomatosis with polyangiitis diagnosis is thought to be difficult in the prodromic phase, but should not be missed in vasculitic stage, since that early treatment improve the prognosis.
Guillain–Barre syndrome mimicking presentation is previously reported at least in nine cases. First case of EGPA mimicking GBS clinically and electrophysiologically was reported by Ng et al. [13]. Their patient had no recovery with plasmapheresis and was later confirmed to be a case of EGPA (persistent eosinophilia, eosinophilic vasculitis in sural nerve biopsy and positive ANCA). In 1998, Keven et al. [14] reported the next case presented initially with GBS-like neuropathy, later developing ANCA-positive nephrotic syndrome. At least five other cases of EGPA have been mentioned, all with clinic and neurophysiologic studies suggestive of GBS [15],[16],[17],[18]. Antineutrophil cytoplasmic antibody positivity, eosinophilia and treatment failure with intravenous immunoglobulin led to the correct diagnosis. Unlike the mentioned cases course, our case had better prognosis due to early bolus methylprednisolone and cyclophosphamide with no other organ involvement.
Eosinophilic granulomatosis with polyangiitis prognosis depends on the five-factor score (FFS) which includes age >65 years, renal dysfunction, cardiac symptoms, gastrointestinal involvement, absence of nose, ear, and throat involvement. With the presence of one factor, the five-year mortality rate is above 21%, and get to 40% with two or more factors [19]. Interestingly, the outcome is widely better with corticosteroids and immunosuppressants, with five-year survival reaching 90% [20].
Conclusion
We learn from this case that EGPA may be revealed by a GBS mimicking presentation. Neurologic system can be involved in the ANCA negative EGPA. Paraclinical tests should be performed in GBS presentation, leading to concise diagnosis and adequate early treatment. Unlike GBS, EGPA polyneuropathy cases improve with methylprednisolone bolus associated with immunosuppressive treatment (cyclophosphamide).
REFERENCES
1.
Masi AT, Hunder GG, Lie JT, et al. The American College of Rheumatology 1990 criteria for the classification of Churg-Strauss syndrome (allergic granulomatosis and angiitis). Arthritis Rheum 1990;33(8):1094–100. [CrossRef]
[Pubmed]

2.
Vaglio A, Buzio C, Zwerina J. Eosinophilic granulomatosis with polyangiitis (Churg-Strauss): State of the art. Allergy 2013;68(3):261–73. [CrossRef]
[Pubmed]

3.
Watts RA, Lane S, Scott DGI. What is known about the epidemiology of the vasculitides? Best Pract Res Clin Rheumatol 2005;19(2):191–207. [CrossRef]
[Pubmed]

4.
Moosig F, Bremer JP, Hellmich B, et al. A vasculitis centre based management strategy leads to improved outcome in eosinophilic granulomatosis and polyangiitis (Churg-Strauss, EGPA): Monocentric experiences in 150 patients. Ann Rheum Dis 2013;72(6):1011–7. [CrossRef]
[Pubmed]

5.
Piram M, Maldini C, Mahr A. Effect of race/ethnicity on risk, presentation and course of connective tissue diseases and primary systemic vasculitides. Curr Opin Rheumatol 2012;24(2):193–200. [CrossRef]
[Pubmed]

6.
Vaglio A, Casazza I, Grasselli C, Corradi D, Sinico RA, Buzio C. Churg–Strauss syndrome. Kidney Int 2009;76(9):1006–11. [CrossRef]
[Pubmed]

7.
8.
Cattaneo L, Chierici E, Pavone L, et al. Peripheral neuropathy in Wegener's granulomatosis, Churg-Strauss syndrome and microscopic polyangiitis. J Neurol Neurosurg Psychiatry 2007;78(10):1119–23. [CrossRef]
[Pubmed]

9.
Sinico RA, Di Toma L, Maggiore U, et al. Prevalence and clinical significance of antineutrophil cytoplasmic antibodies in Churg-Strauss syndrome. Arthritis Rheum 2005;52(9):2926–35. [CrossRef]
[Pubmed]

10.
Mahr A, Moosig F, Neumann T, et al. Eosinophilic granulomatosis with polyangiitis (Churg-Strauss): Evolutions in classification, etiopathogenesis, assessment and management. Curr Opin Rheumatol 2014;26(1):16–23.
[Pubmed]

11.
Nagashima T, Cao B, Takeuchi N, et al. Clinicopathological studies of peripheral neuropathy in Churg-Strauss syndrome. Neuropathology 2002;22(4):299–307. [CrossRef]
[Pubmed]

12.
Sablé-Fourtassou R, Cohen P, Mahr A, et al. Antineutrophil cytoplasmic antibodies and the Churg- Strauss syndrome. Ann Intern Med 2005;143(9):632–8. [CrossRef]
[Pubmed]

13.
Ng KK, Yeung HM, Loo KT, Chan HM, Wong CK, Li PC. Acute fulminant neuropathy in a patient with Churg-Strauss syndrome. Postgrad Med J 1997;73(858):236–8. [CrossRef]
[Pubmed]

14.
Keven K, Nergisoğlu G, Ertürk S, et al. A case of Guillain-Barré syndrome complicated by nephrotic syndrome and p-ANCA positivity. Nephron 1998;80(3):361–2. [CrossRef]
[Pubmed]

15.
Riva N, Cerri F, Butera C, et al. Churg Strauss syndrome presenting as acute neuropathy resembling Guillain Barré syndrome: Case report. J Neurol 2008;255(11):1843–4. [CrossRef]
[Pubmed]

16.
Djukic M, Schmidt H, Mazurek C, König F, Schweyer S, Nau R. A patient with Churg-Strauss syndrome presenting as Guillain-Barré syndrome. [Article in German]. Nervenarzt 2008;79(4):457–61. [CrossRef]
[Pubmed]

17.
De Toni Franceschini L, Amadio S, Scarlato M, et al. A fatal case of Churg-Strauss syndrome presenting with acute polyneuropathy mimicking Guillain-Barré syndrome. Neurol Sci 2011;32(5):937–40. [CrossRef]
[Pubmed]

18.
Camara-Lemarroy CR, Infante-Valenzuela A, Villareal-Montemayor HJ, Soto-Rincon CA, Davila-Olalde JA, Villareal-Velazquez HJ. Eosinophilic granulomatosis with polyangiitis presenting as acute polyneuropathy mimicking Guillain-Barre syndrome. Case Rep Neurol Med 2015;2015:981439. [CrossRef]
[Pubmed]

19.
Guillevin L, Pagnoux C, Seror R, et al. The five-factor score revisited: Assessment of prognoses of systemic necrotizing vasculitides based on the French Vasculitis Study Group (FVSG) cohort. Medicine (Baltimore) 2011;90(1):19–27. [CrossRef]
[Pubmed]

20.
Pagnoux C, Guilpain P, Guillevin L. Churg-Strauss syndrome. Curr Opin Rheumatol 2007;19(1):25–32. [CrossRef]
[Pubmed]

SUPPORTING INFORMATION
Author Contributions
Mohamed Hamid - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Youssef Benmoh - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Cedrick Moussavou - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Aziz Ahizone - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ayoub Bakal - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Mohamed Ajamate - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Amal Satte - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ahmed Bourazza - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Guarantor of SubmissionThe corresponding author is the guarantor of submission.
Source of SupportNone
Consent StatementWritten informed consent was obtained from the patient for publication of this article.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Conflict of InterestAuthors declare no conflict of interest.
Copyright© 2022 Mohamed Hamid et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.