|

|
 |
|
Case Report
| ||||||
| Benign reactive lesion with atypical mitosis: New example of an old story | ||||||
| Hua Zhong1, 2, Marina Chekmarva1, 2, Malik Deen2, Michael May2, Steven Deak3, Nicola Barnard2 | ||||||
|
1MD, PhD, Rutgers Cancer Institute of New Jersey, New Brunswick, New Jersey, United States.
2MD, Department of Pathology and Laboratory Medicine, Rutgers Robert Wood Johnson Medical School, New Brunswick, New Jersey, United States. 3MD, Department of Surgery, Rutgers Robert Wood Johnson Medical School, New Brunswick, New Jersey, United States. | ||||||
| ||||||
|
[HTML Abstract]
[PDF Full Text]
[Print This Article]
[Similar article in Pumed] [Similar article in Google Scholar] 
|
| How to cite this article: |
| Zhong H, Chekmareva M, Deen M, May M, Deak S, Barnard N. Benign reactive lesion with atypical mitosis: New example of an old story. International Journal of Case Reports and Images 2014;5(3):235–239. |
|
Abstract
|
|
Introduction:
Ischemic fasciitis (atypical decubital fibroplasia) is a benign reactive lesion that may mimic mesenchymal malignancy. Atypical mitosis has never been reported in such a lesion, which adds an additional challenge to differential diagnosis.
Case Report: A 94-year-old chronically immobile and debilitated female presented with a slightly painful and rapidly growing mass over her right back. Based on the clinical and histological features of the lesion, the diagnosis of ischemic fasciitis, a benign reactive lesion of the deep dermis, was made. Active mitosis and atypical mitotic figures were demonstrated in the lesion. Conclusion: Ischemic fasciitis may show atypical mitotic figures that can mislead differential diagnosis. | |
|
Keywords:
Ischemic fasciitis, Atypical decubital fibroplasia, Atypical mitosis, Soft tissue
| |
|
Introduction
|
|
In 1892, based on cancer research papers published in the early 20th century, Dr. Stroebe discovered asymmetrical division, or atypical mitosis, in nonmalignant processes with reactive or regenerative changes. Others, however, argued against his discovery and believed that atypical mitosis could only occur in carcinomas, sarcomas or unusual inflammatory reaction in a Cysticercus rat model of which cells with atypical mitosis eventually resulted in malignancy. [1] After over one century, a general consensus has been reached in the surgical pathology society, stating that an atypical mitotic figure may indicate a malignant process while it can also be seen in benign lesions. For example, atypical mitotic figures can be occasionally found in granulation tissue following ionizing-radiation exposur, ischemic colitis, long standing ulcerative colitis, gastric intestinal metaplasia, endometrium associated with chorionic tissue effect, benign cutaneous lesions induced by taxane therapy, and abnormal but non-neoplastic astrocytes. [2] [3] [4] [5] [6] [7] [8] Herein, a rare case of ischemic fasciitis, also named as atypical decubital fibroplasia, is reported, which shows typical clinical and histological features, except that atypical mitosis is evident. |
|
Case Report
|
|
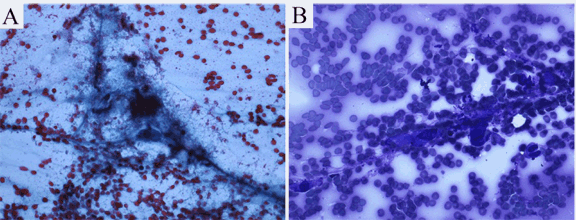
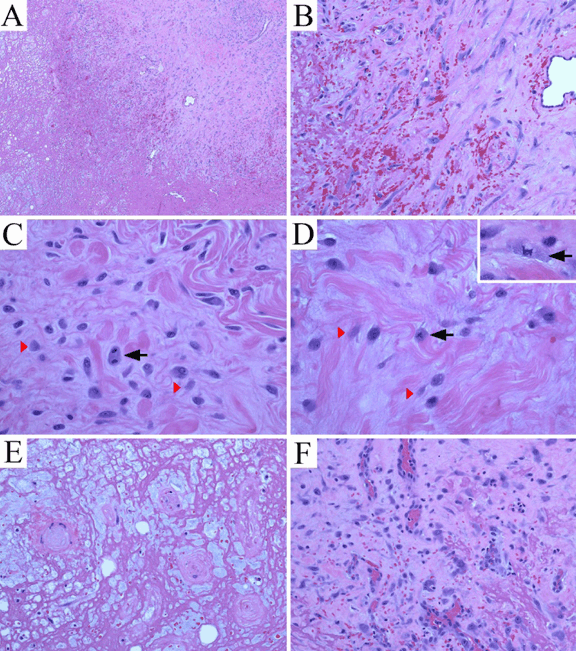
The patient was a 94-year-old female who was chronically immobile and debilitated with a history of locally recurrent basal cell carcinoma (left upper lip and face, up to 1.1 cm in diameter) and superficially invasive cutaneous squamous cell carcinoma (right cheek, 0.8 cm in diameter) of the head and neck region. These cutaneous carcinomas were resected with negative margins. The patient presented with a slightly painful and rapidly growing mass over her right back for two months. She denied history of trauma or prior surgical procedure to the region. On physical examination, the skin surface on the right scapular and axillary regions appeared unremarkable. A 3x3.5 cm slightly firm mass was palpated at the lateral edge of the right scapula at right axillary level. The mobility of the mass was limited while the overlying skin was mobile. No other masses or any signs of lymphadenopathy were present. Results of basic laboratory tests were within normal limits. Since clinical and laboratory findings did not suggest infection, a soft tissue sarcoma or a metastatic lesion was suspected. Fine-needle aspiration (FNA) was performed, and was followed by surgical excision of the mass with skin sparing. The FNA specimen (Figure 1A-B) was basically hypocellular with a background of red blood cells. Smears showed loose aggregates of cells grouped around delicate, branching vascular networks in addition to scant loose cells. Individual cellular constituents included a mixture of spindle shaped fibroblast or myofibroblast-like cells with ample cytoplasm that tended to taper at the ends and with plump ganglion-like cells. Nuclei were large and often dark on smears. In addition to the cellular component, the aspirates demonstrated a background of granular-serous materials and occasional inflammatory cells. The latter included lymphocytes, foamy macrophages and rare neutrophils. Collagen micro-fragments were occasionally seen. Cell block showed fragments of granulation tissue or necrotic tissue with small cysts, which was basically similar histology as demonstrated in the resection specimen. (Figure 2A-F) An FNA cytopathology was interpreted as an atypical spindle cell lesion, and deferred to tissue diagnosis. The surgically excised specimen grossly revealed an irregular, slightly firm, pink-tan and ill-defined mass, 3.5x3.0x2.5 cm in size. Cut surfaces were solid, variegated, red-tan, focally hemorrhagic and myxoid. Histologically, the lesion was situated in the deep subcutis adjacent to superficial skeletal muscle layer. The lesion consisted of a distinct zonal configuration with a central zone of remarkable fibrinoid necrosis forming small cystic and spongy architectures. (Figure 2A) The fibrinoid necrosis involved adipose tissue as well as small vessels of which the lumens became obliterated. (Figure 2E) At the periphery of the lesion was an irregular and thick layer of granulation tissue with vascular proliferation and prominent proliferating endothelial cells. The area was admixed with plump ganglion-like cells. (Figure 2C-D) in a background of collagenous or myxoid stroma. The ganglion-like cells showed enlarged eccentric nuclei with vesicular chromatin, prominent centrally located nucleoli, and abundant eosinophilic cytoplasm. Fairly often, smaller cells with similar cytoplasmic features were shown with lightly smudged nuclei or occasionally with no appreciable chromatin materials. (Figure 2C, D) These cells demonstrated strongly diffuse immunoreactivity for vimentin, were weakly for CD68 (cytoplasmic), weakly focal for CD31, but were completely negative for pan-cytokeratin and S100. The mitotic figure count equaled 15 per 50 high power fields (40x objective and 10x ocular). Two atypical mitotic figures were demonstrated, (Figure 2D) a tripolar and a multipolar. Scattered mononucleated inflammatory cells and rare polymorphonuclear leukocytes were also seen. Focus of granulation tissue with thin wall vessels characterized by margination and extravasation of inflammatory cells was noted. (Figure 2F) Foci of extravasated erythrocytes were frequently evident, mostly located in association with granulation tissue immediately adjacent to the necrotic zone. (Figure 2A-B) The lesion was non-encapsulated, generally hypocellular and with an ill-defined margin. Local induration was noted on the surgical site at one month follow-up. The patient was followed-up again at three and six months post-surgery with completely recovery. Overall findings favored a reactive process— an entity with so-called ischemic fasciitis/atypical decubital fibroplasia that may show active mitosis, whereas atypical mitotic figures have never been reported in such a lesion. |
|
|
|
|
|
Discussion
|
|
Ischemic fasciitis is a rare, but distinctive entity. It is a benign reactive lesion that usually occurs in elderly immobile patients in the bony prominence overlying connective tissue of weight-bearing areas. [9] The pathogenesis of ischemic fasciitis is hypothetically related to intermittent ischemia with resultant fibrinoid necrosis and regenerative changes. [9] The hypothetical pathogenesis does not appear to have yet been reproduced and confirmed in any animal models. The main pitfall in diagnosing the lesion is to clinically and pathologically mistake it for a malignancy of mesenchymal origin. [9] [10] Our case regarding the presentation of atypical mitotic figures in particular raises the consideration of malignancy. The entire histological context, however, does not appear to fit a malignant process. The characteristic clinical and histologic features of ischemic fasciitis have been described in several case reports and case series since 1992. [9] The histological features of ischemic fasciitis partly overlap with but generally show less cellularity than other fasciitis-type or inflammatory myofibroblastic lesions [9, 10]. Ischemic fasciitis is thought to represent a constellation of fibroblastic or myofibroblastic proliferation, and it is a good histological example in the spectrum of pseudosarcomatous lesions. [9] Thus far, abnormal or atypical mitosis has never been reported in ischemic fasciitis or atypical decubital fibroplasia. Recognizing its typical clinical and histological features is critical in differential diagnosis. As in the current case, although atypical mitotic figures are present, the overall characteristic clinical and histological features favor the diagnosis of ischemic fasciitis. Atypical or abnormal mitotic figure is one of the morphological changes that imply anaplasia or lack of differentiation. [11] However, demonstrating atypical mitotic figures in a benign reactive lesion is not something new, but is rare finding in both experimental and surgical pathology related to inflammatory or regenerative processes. [1] [2] [3] [4] [5] [6] [7] [8] One may argue that some of above mentioned chronic pathological processes, for example, long standing ulcerative colitis [4] and gastric intestinal metaplasia, [5] are associated with malignant potential. It is true that the role of ulcerative colitis or intestinal metaplasia as pre-neoplastic lesion is well established, associating with various genetic alterations or chromosomal instability. [12] [13] It is also true that some of but not all these clinical cases will develop malignant tumors. The fact is that there is a lack of or it is difficult to perform clinical studies that support direct malignant association of which a reactive lesion with atypical mitosis eventually develops into a malignant tumor. Nevertheless, ischemic fasciitis is classified as a reactive lesion with unique clinical scenario that is not associated with any malignant behavior. Our current case provides a new example to the old story of which atypical mitosis occurs in a benign reactive lesion. The mechanisms of atypical mitosis are very complicated, and its biological significance is largely context-dependent. In the early studies reviewed by Dr. Mendelsohn, [1] atypical mitosis has been shown in cultured cells subjected to a variety of physical and chemical treatments. Recent studies have demonstrated that abnormal mitosis and DNA damage are reciprocal processes, and that abnormal mitosis can even potentially facilitate the activation of oncogenic stimuli that may promote transformation. [14] The occurrence of atypical mitotic figures, especially multipolar mitosis, is closely associated with centrosome defects and chromosome missegregation. [15] Chromosome missegregation is physically and chemically inducible in primary human fibroblasts [16] and immortalized retinal pigment epithelial cells [17] at a rate lower than neoplastic cells. In addition, induced mitotic defects or chromosome missegregation can be transient and dynamic. [17] [18] The above lines of evidence suggest that chromosomal alterations or atypical mitotic figures can be induced in specific conditions, and that not all atypical mitotic events lead to malignant transformation. In other words, atypical mitosis does not equal to malignancy, although a majority of them do. |
|
Conclusion
|
|
Ischemic fasciitis/atypical decubital fibroplasia may show atypical mitotic figures that can mislead differential diagnosis. This report adds one more example to the benign reactive lesions with atypical mitosis. |
|
References
|
|
|
[HTML Abstract]
[PDF Full Text]
|
|
Author Contributions
Hua Zhong – Substantial contributions to conception and design, Acquisition of data, Analysis and interpretation of data, Drafting the article, Critical revision of the article, Final approval of the version to be published Marina Chekmareva – Substantial contributions to Acquisition of data, Analysis and interpretation of data, Critical revision of the article, Final approval of the version to be published Malik Deen – Substantial contributions to Acquisition of data, Analysis and interpretation of data, Critical revision of the article, Final approval of the version to be published Michael May – Substantial contributions to Acquisition of data, Analysis and interpretation of data, Critical revision of the article, Final approval of the version to be published Steven Deak – Substantial contributions to Acquisition of data, Analysis and interpretation of data, Critical revision of the article, Final approval of the version to be published Nicola Barnard – Substantial contributions to Acquisition of data, Analysis and interpretation of data, Critical revision of the article, Final approval of the version to be published |
|
Guarantor of submission
The corresponding author is the guarantor of submission. |
|
Source of support
None |
|
Conflict of interest
Authors declare no conflict of interest. |
|
Copyright
© Hua Zhong et al. 2014; This article is distributed the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any means provided the original authors and original publisher are properly credited. (Please see Copyright Policy for more information.) |
|
|