|

|
 |
|
Case Report
| ||||||
| Dual causes of multiple myeloma | ||||||
| Zaw Min1, Zipporah Krishnasami2, William J Cook3, J Martin Rodriguez4 | ||||||
|
1MD, Infectious Diseases Fellow, Division of Infectious Diseases, University of Alabama, Birmingham, Alabama, USA.
2MD, Assistant Professor, Division of Nephrology, University of Alabama, Birmingham, Alabama, USA. 3MD, PhD, Emeritus Professor, Department of Pathology, University of Alabama, Birmingham, Alabama, USA. 4MD, FACP, Associate Professor, Division of Infectious Diseases, University of Alabama, Birmingham, Alabama, USA. | ||||||
| ||||||
|
[HTML Abstract]
[PDF Full Text]
[Print This Article]
[Similar article in Pumed] [Similar article in Google Scholar] 
|
| How to cite this article: |
| Min Z, Krishnasami Z, Cook WJ, Rodriguez JM. Dual causes of multiple myeloma. International Journal of Case Reports and Images 2013;4(9):489–493. |
|
Abstract
|
|
Introduction:
Patients infected with human immunodeficiency virus (HIV) infection have been living longer secondary to dramatic improvements in their immune status because of highly active antiretroviral therapy (HAART). Consequently, there is an increasing incidence of non-AIDS defining malignancies and chronic diseases in HIV-infected individuals. The hepatitis C virus (HCV) co-infection is highly prevalent in patients with HIV infection.
Case Report: We report a patient with HIV and HCV co-infection who presented with multiple myeloma, and explore literature looking for a plausible causal association between multiple myeloma, HIV and HCV infections. Conclusion: Multiple myeloma is not a commonly associated malignancy with HIV and/or HCV infection although hyperglobulinemia is often associated. Clinicians should be aware that multiple myeloma may occur as a non-AIDS defining cancer in HIV-infected individuals and/or as an extra-hepatic manifestation in HCV-infected patients. | |
|
Keywords:
Multiple myeloma, Human immunodeficiency virus (HIV), Acquired immune deficiency syndrome (AIDS), Hepatitis C infection
| |
|
Introduction
| ||||||
|
Human immunodeficiency virus (HIV)-infected patients have benefited from dramatic improvements in prognosis and life expectancy because of effective highly active antiretroviral therapy (HAART). The incidence of AIDS-defining neoplasms has consequently decreased. [1] However, non-AIDS defining malignancies and chronic diseases are increasingly being reported in HIV-infected populations. [1] [2] [3] Chronic hepatitis C virus (HCV) infection is highly prevalent in HIV-infected patients due to similarities in the epidemiology. Hepatitis C virus infected patients frequently develop chronic liver disease, but extrahepatic complications have also been reported including HCV-associated B cell tumors. [4] [5] We describe a patient with HIV and HCV co-infection who developed IgG lambda light chain multiple myeloma. We review literature for a possible causal association between multiple myeloma, HIV and HCV infection. | ||||||
|
Case Report
| ||||||
|
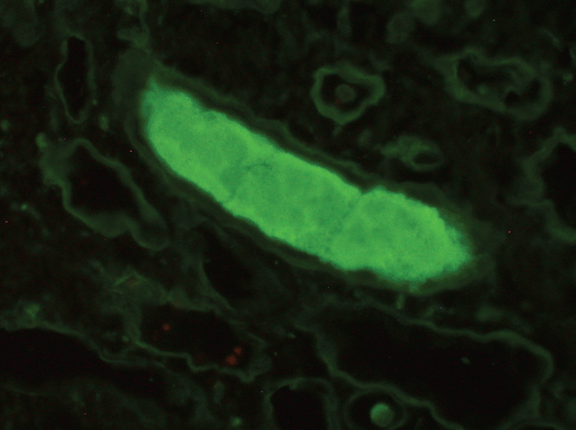
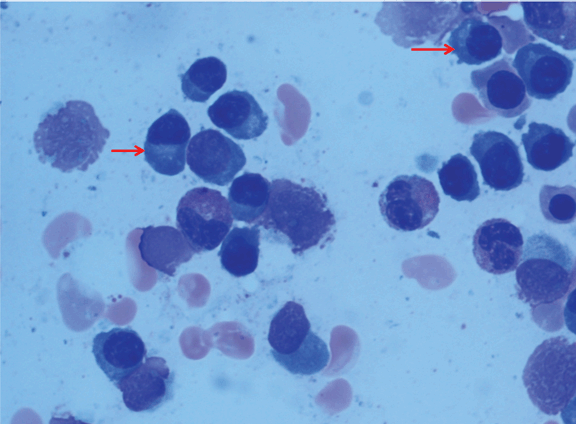
A 47-year-old African-American female co-infected with HIV and HCV presented to our HIV clinic for routine follow-up. She had been diagnosed with HIV and HCV infections eight years before. She had been treated with interferon and ribavirin for HCV infection, but she did not complete therapy due to side effects from interferon. Her HIV infection was well-controlled with anti-retroviral therapy (zidovudine, lamivudine and efavirenz) with CD4 count of 1257 cells/µL and HIV-RNA viral load of less than 20 copies/mL. She complained of fatigue, diffuse arthralgias and unintentional weight loss (8 kg in two months). Physical examination was unremarkable. Her laboratory studies showed normochromic and normocytic anemia with a hemoglobin of 10 g/dL (normal range 10.3–15.2 g/dL), elevated serum creatinine at 2.5 mg/dL (normal range 0.4–1.2 mg/dL), and high globulin gap with a serum total protein of 9.6 g/dL (normal range 6.0–7.9 g/dL) and a serum albumin of 3.3 g/dL (normal range 3.5–5.0 g/dL). An extensive work-up to evaluate the etiology for renal dysfunction was undertaken. A renal sonogram revealed normal-sized kidneys. A 24-hour urine collection showed 2.1 g of protein. Anti-nuclear antibodies and complement levels were normal and HCV-RNA viral load was 20 million copies/mL with HCV genotype 1a. Serum cryoglobulin was negative. Serum and urine protein electrophoreses showed a monoclonal spike (4.26 g/dL) and immunofixation electrophoreses demonstrated monoclonal IgG with elevated free lambda light chains. Serum free monoclonal lambda light chain was elevated at 3500 mg/L (normal range 5.7 – 26.3 mg/L). Skeletal survey was negative. The patient underwent a renal biopsy which revealed light chain casts in the renal tubules, (Figure 1A-B) confirmed as monoclonal lambda light chain on immunofluorescence. (Figure 2) There was no evidence of HIV-associated nephropathy (HIVAN). A bone marrow biopsy showed more than 45% of plasma cell infiltration. (Figure 3) After the diagnosis of IgG lambda light chain multiple myeloma was made, she was treated with nine cycles of initial induction chemotherapy (bortezomib and dexamethasone) followed by autologous hematologic stem cell transplant per the institutional treatment protocol. Subsequently, her serum creatinine improved to 1.1 mg/dL. The patient declined anti-HCV therapy because of her previous intolerance to interferon treatment. | ||||||
|
| ||||||
|
| ||||||
| ||||||
|
Discussion
| ||||||
|
The HIV/AIDS cancer match study, one of the largest epidemiological studies conducted in the United States (US), linked 15 population-based HIV/AIDS and cancer registries in the US. The study analyzed 413,080 HIV-infected persons in 34 US states from 1991 through 2005. During that period, an estimated 79,656 cancers occurred in the AIDS population. It was observed that the incidence of the AIDS-defining malignancies declined markedly over that 14-year period, whereas non-AIDS-defining neoplasms became the predominant type of cancer in HIV-infected persons during the HAART era. Multiple myeloma is one of those non-AIDS-defining cancers whose incidence has increased recently in HIV-infected individuals. [1] The HIV-infected patients can present with a range of plasma cell disorders, from benign polyclonal hypergammaglobulinemia, or indeterminate monoclonal gammopathy of unknown significance (MGUS) to malignant plasma cell dyscrasias. [6] Multiple myeloma is usually not recognized as a malignancy that is associated with HIV infection. There have been only about 50 reported cases of HIV-infected patients with multiple myeloma in literature since the first case was reported in 1983. [7] Multiple myeloma has also been reported as the initial clinical manifestation of HIV/AIDS infection. [8] The pathogenesis of multiple myeloma in HIV-infected patients is multifactorial. (Figure 4) There are at least two major proposed mechanisms:
Another postulated factor is interleukin-6 (IL-6), secreted from bone marrow stromal cells, which stimulates the growth of plasma cells as a paracrine mechanism. Human Herpes Virus-8 (HHV-8) has the ability to produce viral IL-6 (vIL-6), a human homolog of growth factor for plasma cells, which may perpetuate the growth of neoplastic plasma cells in HIV-infected individuals who are co-infected with HHV-8. [11] The association of HCV infection with multiple myeloma is more controversial. Among the hematological disorders, monoclonal gammopathies rarely occur in patients with chronic HCV infection. It is speculated that HCV is lymphotropic and the mechanism which contributes to the pathogenesis of B cell non-Hodgkin lymphomas (NHL) may also play a role in the development of multiple myeloma in HCV-infected populations. (Figure 4) [12] Thus, it is a relatively weak association between HCV infection and multiple myeloma. It is also of interest to note that monoclonal gammopathy, if present, is more prevalent in patients with HCV genotypes 2a and 2c. [13] To the best of our knowledge, this is the first reported case of multiple myeloma in a patient with HIV-HCV co-infection. There are several unique characteristics in our patient. First of all, she is older than the average age (33 years) of HIV-positive patients with multiple myeloma. Secondly, the clinical course of multiple myeloma in our patient was relatively less aggressive than the course usually noted in HIV-infected patients. Thirdly, her HIV infection was well-controlled, and there was no parallel progression of multiple myeloma with HIV infection to AIDS. Lastly, her HCV genotype was 1a, which is not one of the most commonly observed genotypes (2a/2c) in HCV-infected patients with multiple myeloma. | ||||||
| ||||||
|
| ||||||
|
Conclusion
| ||||||
|
In conclusion, chronic infection with human immunodeficiency virus (HIV) and/or hepatitis C virus (HCV) appears to increase the risk of developing multiple myeloma, although their oncogenic role has been less established. Our case highlights that dual HIV and HCV infections may possibly have an additive effect that drives this oncogenic process leading to multiple myeloma. Further research is warranted to establish a pathogenic causal relationship. Nonetheless, providers should keep in mind that multiple myeloma may occur in HIV-mono-infected or HIV-HCV co-infected patients, especially when serum gammopathy is present. | ||||||
|
References
| ||||||
| ||||||
|
[HTML Abstract]
[PDF Full Text]
|
|
Author Contributions
Zaw Min – Conception and design, Acquisition of data, Analysis and interpretation of data, Drafting the article, Critical revision of the article, Final approval of the version to be published Zipporah Krishnasami – Conception and design, Acquisition of data, Analysis and interpretation of data, Drafting the article, Critical revision of the article, Final approval of the version to be published William J Cook – Conception and design, Acquisition of data, Analysis and interpretation of data, Drafting the article, Critical revision of the article, Final approval of the version to be published Martin Rodriguez – Conception and design, Acquisition of data, Analysis and interpretation of data, Drafting the article, Critical revision of the article, Final approval of the version to be published |
|
Guarantor of submission
The corresponding author is the guarantor of submission. |
|
Source of support
None |
|
Conflict of interest
Authors declare no conflict of interest. |
|
Copyright
© Zaw Min et al. 2013; This article is distributed the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any means provided the original authors and original publisher are properly credited. (Please see Copyright Policy for more information.) |
|
|