|

|
 |
|
Case Report
| ||||||
| Octreotide induced thrombocytopenia | ||||||
| Haroon Yousaf1, Irfan Saddique2 | ||||||
|
1Theda Clark Hospital, Neenah, WI, USA.
2Interfaith Medical Center, Brooklyn, New York, USA. | ||||||
| ||||||
|
[HTML Abstract]
[PDF Full Text]
[Print This Article]
[Similar article in Pumed] [Similar article in Google Scholar] 
|
| How to cite this article: |
| Yousaf H, Saddique I. Octreotide induced thrombocytopenia. International Journal of Case Reports and Images 2012;3(11):14–16. |
|
Abstract
|
|
Introduction:
Thrombocytopenia due to octreotide is a rare but known phenomenon. Only three cases of octreotide induced thrombocytopenia have been reported so far.
Case Report: Here we report a case of a 76-year-old diabetic male who was brought to the emergency room after being found unconscious. In the ER, his blood glucose level was 43 mg/dL. He was taking glyburide 5 mg by mouth daily prior to admission. Escalated dextrose infusion with repeated doses of D50W failed to sustain his blood glucose, which remained in the range of 30 to 50 mg/dL. Salvage treatment with intravenous octreotide was implemented successfully; only one dose of D50W was required after octreotide initiation and blood glucose normalized within several hours. However, a significant drop in patient's platelet count was noted after initiation of octreotide therapy, that persisted until the drug was discontinued. Rapid recovery was observed after discontinuation of octreotide. Conclusion: High degree of suspicion is warranted on the part of physicians to suspect octreotide as a causative factor for thrombocytopenia. We suggest that octreotide be discontinued when thrombocytopenia occur, once other known causes of thrombocytopenia have been excluded. | |
|
Key Words:
Octreotide, Thrombocytopenia, Drug toxicity
| |
|
Introduction
| ||||||
|
Thrombocytopenia due to octreotide is a rare but known phenomenon. Only three cases of octreotide induced thrombocytopenia have been reported so far. [1] [2] [3] The diagnosis of this critical condition is based on clinical suspicion and is a diagnosis of exclusion. We report a case of octreotide-induced reversible thrombocytopenia in a diabetic patient who presented with hypoglycemia. | ||||||
|
Case Report
| ||||||
|
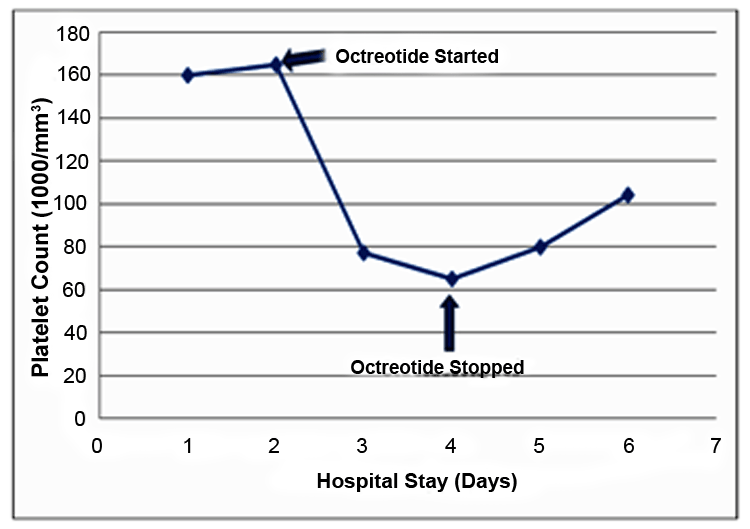
A 76-year-old male was in his usual state of health until three days prior to presentation when he had an episode of documented hypoglycemia with loss of consciousness. His medical history consisted of diabetes mellitus, for which he was taking glyburide 1 mg by mouth daily for two years, prior to admission. The day of the event he went on a walk with wife. Six hours after his breakfast of cereal he began to feel lightheaded and then lost consciousness, which was witnessed by his wife. The patient has no recollection of the event, but denies any preceding vision changes, warmth, sweating anxiety, weakness, nausea or palpitations. His wife denies witnessing any muscle contractions or seizure activity during the episode. Emergency medical services were called in. When the paramedics arrived, the patient had a serum glucose value of 54 mg/dL. He was taken to the emergency room of a local hospital and found to have a serum glucose of 43 mg/dL. He was given one ampule of 50% dextrose, resulting in significant improvement in his mental status. His initial vital signs revealed blood pressure of 111/73 mmHg, heart rate of 99/minute, respiratory rate of 22/minute, and 100% oxygen saturation on room air. His neck was supple. Examination of the lungs revealed bilateral good air entry. Cardiac examination demonstrated normal first and second heart sounds with a regular rhythm and no murmurs. His abdomen was soft and non tender and his extremities were warm and dry. His relevant laboratory findings were hemoglobin 13 g/dL, platelets 160,000/mm3, International Normalized Ratio 1.1, and mean corpuscular volume 80.9 fL/red blood cell. The patient was then admitted to the hospital for further evaluation of his hypoglycemia. Serum sulfonylurea screen was noted to be positive. Hypoglycemia persisted over the next 24 hours during the course of her hospital stay despite continuous infusion of 10% dextrose. Salvage treatment with intravenous continuous octreotide 50 mcg/hr infusion was implemented successfully after 24 hours of his hospital stay; only one dose of D50W was required after octreotide initiation and blood glucose normalized within several hours. Nine hours after, starting octreotide infusion the patient's platelet count had decreased to 77,000/mm3. Peripheral blood smear did not show any abnormalities. As part of his thrombocytopenia work-up, blood cultures, a disseminated intravascular coagulation panel and heparin-induced antiplatelet antibodies were obtained, all of which came back negative. Octreotide was discontinued 16 hours after admission, with a presumptive diagnosis of drug-induced thrombocytopenia. A quick recovery in the patient's platelet count occurred (Figure 1), and he remained stable and was discharged on sixth day after admission with a platelet count of 104,000/mm3. During a follow-up examination after one week patient denied any bleeding or bruising and his platelet count was noted to be 155,000/mm3. The decline in platelet count was only seen after octreotide initiation, with immediate improvement when it was discontinued, leading to our diagnosis of octreotide-induced reversible thrombocytopenia. | ||||||
| ||||||
|
Discussion
| ||||||
|
In its short-acting preparation, octreotide has been used safely in humans since 1998. Thrombocytopenia is an extremely rare side effect of octreotide therapy. Only three cases of this condition have previously been reported in literature. [1] [2] [3] Platelet count typically falls from 50% of the normal value on exposure to the drug and returns to normal after drug withdrawal. Octreotide causes thrombocytopenia most likely through immunologic phenomena, [4] that is, by drug-dependent antibodies causing accelerated platelet destruction by binding to platelet surface glycoproteins. [5] It usually takes one week for platelets to recover following drug discontinuation. [6] | ||||||
|
Conclusion
| ||||||
|
We suggest that octreotide be discontinued when thrombocytopenia occur, once other known causes of thrombocytopenia have been excluded. | ||||||
|
References
| ||||||
| ||||||
|
[HTML Abstract]
[PDF Full Text]
|
|
Author Contributions:
Haroon Yousaf – Substantial contributions to conception and design, Acquisition of data, Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published Irfan Saddique – Substantial contributions to conception and design, Analysis and interpretation of data, Drafting the article, Final approval of the version to be published |
|
Guarantor of submission:
The corresponding author is the guarantor of submission. |
|
Source of support:
None |
|
Conflict of interest:
Authors declare no conflict of interest. |
|
Copyright:
© Haroon Yousaf et al. 2012; This article is distributed the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any means provided the original authors and original publisher are properly credited. (Please see Copyright Policy for more information.) |
|
|