| Table of Contents |  |
|
Case Report
|
| Recurrent intestinal type sinonasal adenocarcinoma treated with FOLFOX6 chemotherapy: Case report and review of literature |
| Mateya E Trinkaus1, Andrew Coleman2, Ieta D'Costa2, Elizabeth Sigston3, Danny Rischin1 |
|
1Peter MacCallum Cancer Centre, Department of Medical Oncology, East Melbourne, Victoria, Australia.
2Peter MacCallum Cancer Centre, Department of Radiation Oncology, East Melbourne, Victoria, Australia. 3Monash Medical Centre, Department of Otolaryngology, Head and Neck Surgery, East Bentleigh, Victoria, Australia. |
|
doi:10.5348/ijcri-2012-08-158-CR-5
|
|
Address correspondence to: Danny Rischin Peter MacCallum Cancer Centre, Department of Medical Oncology Locked Bag No. 1, A Beckett St. East Melbourne, VIC 8006 Australia Phone: 011 (03) 9656 1804; Fax: 011 (03) 9656 1408 Email: danny.rischin@petermac.org |
|
[HTML Abstract]
[PDF Full Text]
|
| How to cite this article: |
| Trinkaus ME, Coleman A, D'Costa I, Sigston E, Rischin D. Recurrent intestinal type sinonasal adenocarcinoma treated with FOLFOX6 chemotherapy: Case report and review of literature. International Journal of Case Reports and Images 2012;3(8):17–20. |
|
Abstract
|
|
Introduction:
Sinonasal intestinal-type adenocarcinoma is a rare malignancy originating most commonly from the ethmoid sinuses. Morphologically, this cancer tends to resemble intestinal adenocarcinoma with immunohistologic staining mirroring what would be expected for a gastrointestinal malignancy. Definitive treatment involves surgical resection often followed by radiation. In the inoperable or metastatic setting, there is limited evidence for effective chemotherapeutic regimens.
Case Report: A patient with unresectable sinonasal intestinal-type adenocarcinoma. Chemotherapy with 5-FU and oxaliplatin (FOLFOX) was initiated based on the rationale of similar morphology and immunohistochemistry (IHC) of this patient's sinonasal intestinal-type adenocarcinoma and colon adenocarcinoma and the affirmed efficacy of FOLFOX regimens in the metastatic and adjuvant colon cancer settings. The patient had a dramatic partial radiologic response by response evaluation criteria in solid tumors (RECIST) criteria. Conclusion: Intestinal-type sinonasal adenocarcinoma presents a rare and challenging opportunity to select active chemotherapy for unresectable disease. We propose that further investigation of colorectal chemotherapy regimens including FOLFOX in intestinal-type sinonasal adenocarcinoma is warranted. | |
|
Key Words:
Sinonasal adenocarcinoma, Intestinal-type, Oxaliplatin, Treatment
| |
|
Introduction
| ||||||
|
Sinonasal intestinal-type adenocarcinoma is a rare malignancy originating most commonly from the ethmoid sinus. Curative treatment with surgery and radiation is limited by the anatomic complexity and proximity of these tumors to sensitive neural structures, resulting in a high risk of local relapse. No standard chemotherapy approaches exist in the adjuvant or metastatic settings. Herein, we present a case of recurrent sinonasal intestinal-type adenocarcinoma treated with a chemotherapy regimen approved for colon cancer (FOLFOX6), based on the rationale of similar immunohistochemistry and morphologic appearances with colon adenocarcinoma, resulting in a dramatic partial radiologic response. | ||||||
|
Case Report
| ||||||
|
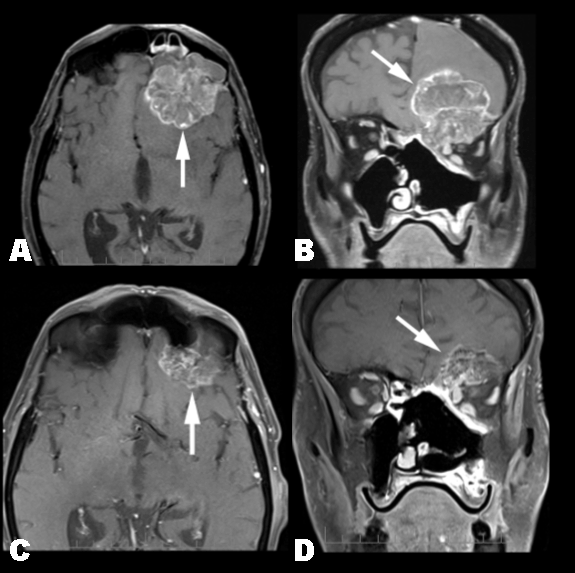
A 65-year-old wood carpenter presented in January 2008 with a several week history of progressive left nasal congestion despite repeated antibiotic treatment. His past medical history was significant for impaired right eye visual acuity since childhood, and a 15 pack year smoking history. The initial MRI brain and examination under anesthesia revealed a bulky bi-lobed T4aN0 tumor centered in the left ethmoid sinus and nasal cavity, extending into sphenoid sinus. There was no evidence of intracranial or perineural extension. Left nasal polyp biopsy confirmed sinonasal adenocarcinoma. The patient underwent lateral rhinotomy, bilateral sphenoidectomies, left maxillectomy and anterior and posterior ethmoidectomies. Histopathology reported an ulcerated, moderately differentiated, colonic-type adenocarcinoma, with clear margins. Adjuvant intensity modulated radiation therapy (60 Gy) was delivered to the surgical bed, with margins limited by proximity to the patient's functioning eye. In March 2010, the patient presented with nasal congestion, left proptosis, diplopia, and reduced left visual acuity. MRI brain showed an inoperable 48x45x48 mm intracranial mass arising from the dura of the left anterior cranial fossa floor, causing mass effect on the left frontal lobe with marked edema, midline shift, and inferior extension into the left orbit. CT and FDG-PET were consistent with the MRI findings. A repeat biopsy revealed moderately to poorly differentiated intestinal-type sinonasal adenocarinoma with immunoreactivity for CK20, CDX-2, and minimal CK7 immunoreactivity. Treatment with FOLFOX6 chemotherapy (oxaliplatin 100 mg/m2; 5-FU 400 mg/m2 bolus followed by 2400 mg/m2 infusion over 46 hours) was initiated based on the rationale of similar morphology and immunohistochemistry (IHC) of this patient's sinonasal intestinal-type adenocarcinoma and colon adenocarcinoma and the affirmed efficacy of FOLFOX regimens in the metastatic and adjuvant colon cancer settings. In total, 12 cycles of FOLFOX6 chemotherapy were administered over six months prior to ceasing treatment on account of treatment related toxicities. The oxaliplatin dose was reduced by 15% after five cycles due to grade 2 peripheral neuropathy. Clinically, the patient had resolution of his nasal congestion, diplopia, and left proptosis, although his left visual acuity remained unchanged. Interim MRIs during treatment showed progressive shrinkage of the primary tumor, reduced mass effect in the left frontal lobe, and resolution of the midline shift and ventricular compression. After 11 cycles of FOLFOX chemotherapy, the primary lesion measured 22.8x17.3x23.3 mm consistent with a partial response by RECIST criteria (Figure 1). Moreover, the majority of the T2 bright tumoral signal resolved suggesting fibrosis. Finally, tumor was no longer detected in the frontal sinus and superior orbital fissure, and there was significant intranasal and left globe tumor regression. Repeat MRI imaging two months after FOLFOX chemotherapy demonstrated progressive disease. Treatment with weekly paclitaxel was given over two months with subsequent further disease progression. Due to a decline in performance status, FOLFOX was not re-initiated, and the patient died six months thereafter. | ||||||
| ||||||
|
Discussion
| ||||||
|
Sinonasal adenocarcinoma is most often classified as being nonintestinal-type and intestinal-type. Intestinal-type adenocarcinoma is a rare malignancy accounting for <20% of sinonasal malignancies. [1] It typically has an indolent growth rate. The diagnosis is often delayed until the tumor is locally advanced, with an associated reduction in prognosis, particularly for T4 tumors with intracranial extension. [2] Intestinal-type sinonasal adenocarcinoma has an IHC profile that mirrors lower gastrointestinal primary adenocarcinoma with expression of CK20, CDX-2, villin, and with variable CK7 expression. [3] Genetic mutations associated with hereditary colon cancer such as loss of expression of one of the mismatch repair enzymes (MLH1, MSH2, MSH3, and MSH6), mutations in the adenomatous polyposis coli or E-cadherin genes, and Kras mutations have not been readily identified in sinonasal adenocarcinomas, but chromosomal abnormalities implicated in the pathogenesis of colorectal adenocarcinoma have been reported in multiple patient series. For example, gene expression profiles have shown a high frequency of chromosomal gains in 5p, 7p, 8q, 12p, and 20q, [4] [5] whereas chromosomal losses have been reported in chromosomes 4, 5q, 8p, 17p and 18q. [4] [5] The exact molecular mechanisms causing intestinal-type sinonasal adenocarcinoma are unknown. Intestinal metaplasia of the respiratory epithelium with increased p53 expression seems to be a precursor for this disease. [3] Complete surgical excision is the mainstay of curative treatment for sinonasal adenocarcinomas, however, the anatomic complexity and close proximity to sensitive neural structures often prevents removal of all macroscopic and subclinical disease. Most patients therefore receive adjuvant radiation. [6] There is no established role for adjuvant chemotherapy. Given the inherent limitations with surgery and radiation, sinonasal adenocarcinoma has a high local recurrence rate of 50%, [2] which is the primary cause of subsequent morbidity. Metastases occur in <10% of patients. Licitra et al. published a phase II trial of 30 patients with intestinal-type adenocarcinoma of the ethmoid sinus treated with induction cisplatin, 5-FU, and leucovorin followed by surgery and radiation. This trial showed a pathologic complete response (pCR) in twelve out of thirty patients which significantly corresponded with the presence of a functional p53 gene (p≤=0.0001), and with improved disease-free survival. [7] The promising pCR rates from induction cisplatin, 5-FU and leucovorin [7] suggest chemosensitivity to this combination, however it is noteworthy that response rates are much lower in the locoregionally recurrent head and neck cancer setting compared to induction response rates prior to surgery and radiotherapy. One case report described a partial radiologic response and complete metabolic response in a patient with an intestinal-type sinonasal adenocarcinoma with pulmonary metastases who received six cycles of TPF (docetaxel 50 mg/m2, cisplatin 60 mg/m2, 5-FU 600 mg/m2 day 1-5) over the course of one year with a survival of 43 months at the time of report submission. [8] | ||||||
|
Conclusion
| ||||||
|
Intestinal-type sinonasal adenocarcinoma presents a rare and challenging opportunity to select active chemotherapy for unresectable disease. We propose that further investigation of colorectal chemotherapy regimens including FOLFOX6 in intestinal-type sinonasal adenocarcinoma is warranted. | ||||||
|
References
| ||||||
| ||||||
|
[HTML Abstract]
[PDF Full Text]
|
|
Author Contributions:
Mateya E Trinkaus – Conception and design, Acquisition of data, Analysis and interpretation of data, Drafting the article, Critical revision of the article, Final approval of the version to be published Andrew Coleman – Conception and design, Acquisition of data, Analysis and interpretation of data, Drafting the article, Critical revision of the article, Final approval of the version to be published Ieta D'Costa – Analysis and interpretation of data, Drafting the article, Critical revision of the article, Final approval of the version to be published Elizabeth Sigston – Analysis and interpretation of data, Critical revision of the article, Final approval of the version to be published Danny Rischin – Conception and design, Acquisition of data, Analysis and interpretation of data, Drafting the article, Critical revision of the article, Final approval of the version to be published |
|
Guarantor of submission:
The corresponding author is the guarantor of submission. |
|
Source of support:
None |
|
Conflict of interest:
Authors declare no conflict of interest. |
|
Copyright:
© Mateya E Trinkaus et al. 2012; This article is distributed the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any means provided the original authors and original publisher are properly credited. (Please see Copyright Policy for more information.) |
|
|