|
 |
|
Case Report
| ||||||
| Peripheral emboli in a patient with atrial fibrillation: A case report | ||||||
| Ismail Tabash1, Alexa S. Riester2, Rebecca Napier1, Joe B. Calkins3 | ||||||
|
1MD, Cardiology Section, Augusta University, 1120 15th Street Augusta, GA 2MD, Emergency Medicine Department, Augusta University,1120 15th Street Augusta, GA 3MD, F.A.C.C., Street Augusta, GA | ||||||
| ||||||
|
[HTML Abstract]
[PDF Full Text]
[Print This Article] [Similar article in Pumed] [Similar article in Google Scholar] 
|
| How to cite this article |
| Tabash I, Riester AS, Napier R, Calkins JB. Peripheral emboli in a patient with atrial fibrillation: A case report. Int J Case Rep Images 2017;8(10):653–658. |
|
ABSTRACT
| ||||||
|
Introduction: Atrial fibrillation is a common arrhythmia that can be associated with a high risk of thromboembolic complications. Ischemic stroke due to involvement of the cerebrovascular circulation is the most common of these complications. Peripheral emboli may not be as easily recognized and the data regarding their risk, incidence and diagnosis is limited. Keywords: Arterial, Atrial fibrillation, Peripheral, Thromboembolism | ||||||
|
INTRODUCTION
| ||||||
|
Atrial fibrillation (AF) is the most common arrhythmia with a prevalence ranging from 1.2–2.8% in patients 60–69 years of age to 7.3–13% in those 80 years or older [1]. Atrial fibrillation is associated with a high risk of thromboembolic complications, significantly contributing to disease morbidity and mortality [2]. Ischemic stroke is the most common thromboembolic complication and may occur as the initial presentation of AF despite appropriate anticoagulation. Data regarding the risk of peripheral embolization is more limited and not as well described in the medical literature. We present the rare case of a patient who presented with simultaneous arterial emboli in two arterial distributions in the setting of atrial fibrillation with rapid ventricular rate. We then review the current literature concerning the incidence, risk, diagnosis and prognosis of peripheral arterial emboli. | ||||||
|
CASE REPORT
| ||||||
|
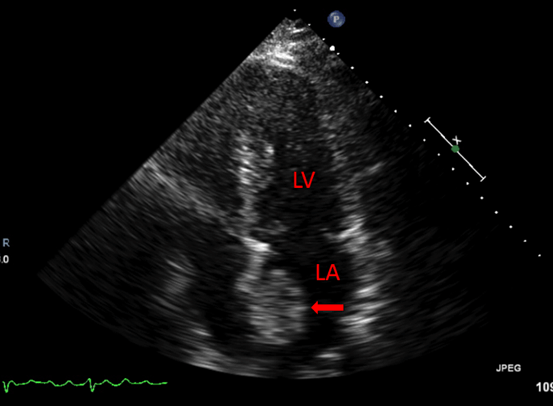
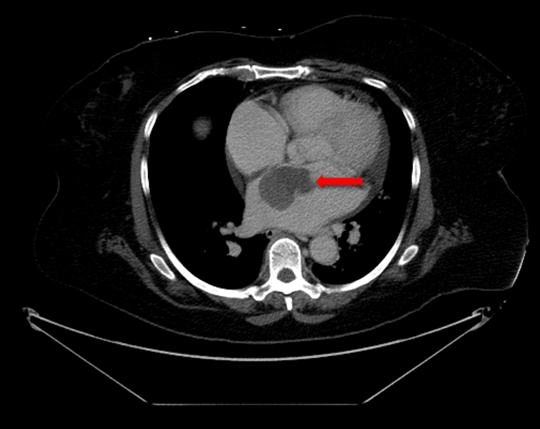
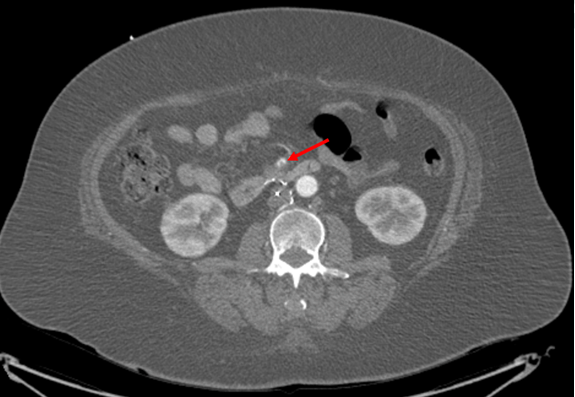
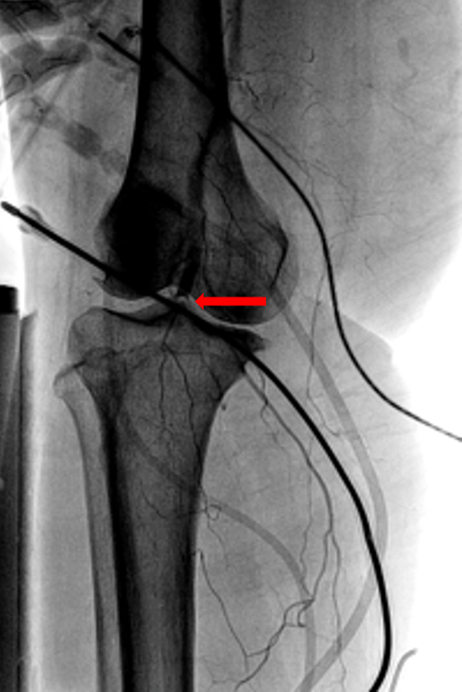
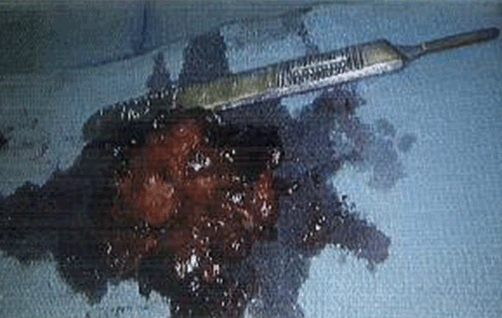
A 66-year-old female with hypertension and hyperlipidemia presented to the emergency department for evaluation of one week of exertional dyspnea and right leg pain. She was a non-smoker and she had no history of peripheral arterial disease, hyperthyroidism or alcohol use. Upon presentation, she was hemodynamically stable. Physical examination was remarkable for irregular tachycardia, hypertension and diminished right pedal arterial pulses. No cardiac murmurs or focal neurological deficits were noted. Telemetry and electrocardiography were consistent with atrial fibrillation with rapid ventricular response. The patient denied a prior history of such arrhythmia. Rate control was achieved with diltiazem infusion and anticoagulation with intravenous heparin was initiated. Echocardiogram showed a left atrial mass adjacent to the interatrial septum suspicious for atrial myxoma (Figure 1). Computed tomography scan showed a left atrial filling defect that enhanced with contrast and measured 4.5x3.3x4.4 cm (Figure 2) as well as a filling defect in the superior mesenteric artery (Figure 3). Lower extremity angiogram demonstrated abrupt cessation of flow consistent with an embolus to the right popliteal artery (Figure 4) and a patent left popliteal artery. Right popliteal arterial thrombectomy was performed. The patient then underwent excision of the left atrial mass. Maze procedure and left atrial appendage ligation. The examination of excised mass revealed an organized thrombus (Figure 5). The postoperative course was uneventful and the patient was discharged on warfarin and metoprolol. | ||||||
| ||||||
| ||||||
| ||||||
| ||||||
| ||||||
|
DISCUSSION
| ||||||
|
Ischemic stroke accounts for 80–90% of embolic events associated with AF whereas peripheral emboli account for 10–20% [3][4]. Current guidelines recommend the utilization of the CHADS2 and CHA2DS2-VASc scores to estimate stroke risk. CHADS2 assigns 1 point each for systolic heart failure with LVEF < 40%, hypertension, age > 75 years and diabetes mellitus and 2 points for prior stroke, transient ischemic attack or arterial embolus. CHA2DS2-VASc assigns 2 points for prior stroke, transient ischemic attack or arterial embolus of for age > 75 years and one point each for heart failure with LVEF <40%, hypertension, diabetes mellitus, vascular disease, age 65–74 years and female gender [2][3][4][5]. Although the clinical significance of the CHADS2 and CHA2DS2-VASc scores to predict stroke risk is well established, it is unclear if these scores have similar ability to predict peripheral embolism risk. Yamamoto et al. [6] investigated the utility of the CHADS2 score in the prediction of peripheral emboli in 117 patients with non-cerebral acute arterial emboli. They found the CHADS2 score to be an unreliable predictor of non-cerebral embolism, with a high risk of non-cerebral occlusion even in patients with a low CHADS2 score [6]. The ATRIA study assessed five risk stratification schemes for their ability to predict atrial fibrillation-related thromboembolism (both cerebral and peripheral emboli) in a large community-based cohort [7]. The authors concluded that these risk stratifications schemes had similar and relatively poor ability to predict thromboembolic risk in persons with non-valvular AF [7]. In a Mayo clinic study published in 2008 [8], 72 patients with peripheral emboli associated with non-valvular AF were randomly compared to 100 patients with stroke in the setting of AF to assess clinical and echocardiographic features determining thromboembolic site. In this study, age >75 years, hypertension and heart failure were associated with a higher incidence of peripheral emboli. Echocardiographic features associated with higher risk included severe left ventricular systolic dysfunction, severe left atrial enlargement, spontaneous echocardiographic contrast and left atrial thrombus [8]. Bekwelem et al. [9] examined the data from four large randomized clinical trials of anticoagulation in AF. Among 37,973 patients and 91,746 patient-years of follow-up, 221 systemic embolic events occurred in 219 subjects. In comparison with stroke patients, those with systemic embolic events were more often smokers, were female, had peripheral arterial disease and had a previous systemic embolic event [9]. In Bekwelem’s analysis, systemic embolic events comprised 11.5% of all clinically recognized thromboembolic events, with an incidence of 0.24 of 100 patient-years compared to an incidence of 1.92 of patient-years for stroke [9]. The most common locations of peripheral emboli were the lower extremity (58%), visceral-mesenteric (31%) and the upper extremity arteries (10%) [9]. An earlier Danish trial published in 2001 [10] analyzed the risk of peripheral emboli in the setting of AF. In this study, 14,917 men and 14,945 women aged 50–89 years diagnosed with AF in the Danish National Hospital Discharge Register from January, 1980 through December, 1993 were followed from the diagnosis of AF until the first diagnosis of a thromboembolic event, death or the end of the study period. In this study, both men (relative risk 4.0, 95% CI: 3.5–4.6) and women (relative risk 5.7, 95% CI: 5.1–6.3) were at increased risk for the occurrence of a peripheral thromboembolic event [10]. The extremities were the most common extracranial location of emboli (61%), followed by the mesenteric arteries (29%), pelvic arteries (9%), aorta (7%) and renal arteries (2%) [10]. Simultaneous emboli in different arterial distributions, as occurred in our patient, are encountered less frequently. In Bekwelem’s analysis [9], simultaneous emboli occurred in only 2 of 219 patients with peripheral emboli. Lee et al. [11] report the case of a patient who presented with simultaneous cerebral and brachial arterial emboli in whom the pain and weakness in his right arm was initially believed to be the result of an acute thromboembolic stroke. Interpretation of the clinical situation may be difficult as a result of atypical symptoms and more subtle signs and because the sequelae of an embolus to a particular arterial distribution may overshadow those occurring as a result of occlusion of another territory. Additionally, because of redundant circulation, the minor clinical consequences of smaller hepatic or splenic infarcts or the lack of acute symptoms with renal arterial emboli, peripheral emboli may be silent or unrecognized [9][12]. As a result, the incidence of peripheral emboli and multiple acute emboli is probably higher than reported whereas the ratio of intracranial to extracranial emboli is likely lower than reported. The symptoms and signs of the more commonly encountered emboli to the arteries of the extremities are more specific than those due to emboli to the visceral organs. The clinical presentation of arterial occlusion involving the extremities includes paresthesia, pain, pallor, pulselessness, poikilothermia and paralysis (the six P’s). Vascular imaging with duplex ultrasonography, computed tomographic angiography (CTA), magnetic resonance angiography (MRA) or standard angiography will confirm the diagnosis. However, anticoagulation should be initiated promptly based on the clinical history and physical exam findings and should not be delayed while awaiting vascular imaging results. Definitive treatment includes catheter-based thrombolytic therapy and/or mechanical embolectomy depending on the duration of ischemia and embolus location and length [13]. The clinical manifestations of emboli to visceral organs can be quite variable and nonspecific depending on the location of the organ affected and the presence or absence of collateral circulation. Early diagnosis is vital to prevent organ infarction and requires a high index of suspicion. Emboli to the mesenteric arteries should be suspected in patients with AF presenting with sudden severe abdominal pain that is out of proportion to the physical exam signs. Diagnosis can be confirmed by vascular imaging including CTA or standard angiography of the mesenteric vasculature. Emergent treatment with anticoagulation and surgical embolectomy are necessary to prevent bowel infarction. Clinical presentation of acute occlusion of the renal arteries is nonspecific and symptoms can be attributed to nephrolithiasis or pyelonephritis. However, acute renal artery occlusion should be suspected in patients with AF and acute unilateral flank pain associated with hematuria and hypertension. Computed tomographic angiography (CTA) or magnetic resonance angiography (MRA) is necessary for the diagnosis. Treatment includes anticoagulation and percutaneous embolectomy. Splenic artery occlusion due to AF is rare. Symptoms are also nonspecific and include left upper quadrant abdominal pain, nausea and vomiting. Diagnosis is confirmed angiographically [14]. Morbidity and mortality increases following a peripheral embolic event. Gosk-Bierska [15] and Sundboll [16] demonstrated a higher rate of thromboembolism among patients with peripheral emboli in the setting of AF compared to those with a stroke and AF. This risk persisted over a follow-up period of 10 years [16]. Patients with a visceral-mesenteric embolus had a significantly higher mortality rate as compared to those with stroke alone [9] over a mean follow-up period of 2.4 years. Mortality risk was also increased to a lesser degree in those with lower extremity and upper extremity emboli over the same period [9]. | ||||||
|
CONCLUSION
| ||||||
|
Although ischemic stroke remains the most common embolic complication of atrial fibrillation (AF), peripheral emboli may also account for 10–20% of embolic events related to this arrhythmia. Peripheral emboli are also associated with significant morbidity and mortality and may go unrecognized unless a high index of clinical suspicion is present. Early diagnosis and therapy for a wide array of embolic events associated with AF is essential to reduce complications and improve clinical outcomes. All patients with AF and peripheral emboli remain at high risk for recurrent embolic events and should receive lifelong anticoagulation not only to prevent recurrence but also to decrease the risk of future stroke. Although the CHADS2 and CHA2DS2-VASc scores are not reliable predictors of the risk of peripheral emboli, it should be emphasized that an arterial thromboembolic event immediately increases these scores to at least 2. These patients are therefore at significantly higher risk for a future thromboembolic stroke and lifelong anticoagulation is required. | ||||||
|
REFERENCES
| ||||||
| ||||||
|
[HTML Abstract]
[PDF Full Text]
|
|
Author Contributions
Ismail Tabash – Substantial contributions to conception and design, Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published Alexa S. Riester – Substantial contributions to conception and design, Drafting the article, Final approval of the version to be published Rebecca Napier – Substantial contributions to conception and design, Drafting the article, Final approval of the version to be published Joe B. Calkins – Substantial contributions to conception and design, Drafting the article, Final approval of the version to be published |
|
Guarantor
The corresponding author is the guarantor of submission. |
|
Source of support
None |
|
Conflict of interest
Authors declare no conflict of interest. |
|
Copyright
© 2017 Ismail Tabash et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information. |
|
|