|
 |
|
Case Report
| ||||||
| Amyloidosis of the colon | ||||||
| Yvonne M. Dawkins1, Barrie Hanchard2, Michael G. Lee3 | ||||||
|
1MB, BS, DM, Lecturer, Department of Medicine, University of the West Indies, Jamaica 2MB, BS, FRCPC, Professor of Pathology, Department of Pathology, University of the West Indies, Jamaica 3MB, BS, DM, FRCPC, FACP, FACG, FRCP, Professor of Medicine, Department of Medicine, University of the West Indies, Jamaica | ||||||
| ||||||
|
[HTML Abstract]
[PDF Full Text]
[Print This Article] [Similar article in Pumed] [Similar article in Google Scholar] 
|
| How to cite this article |
| Dawkins YM, Hanchard B, Lee MG. Amyloidosis of the colon. Int J Case Rep Images 2017;8(9):567–570. |
|
ABSTRACT
|
|
Introduction:
Gastrointestinal amyloidosis is rare in western countries. The most frequent clinical manifestation in all patients with amyloidosis is weight loss and gastrointestinal bleeding. However, diarrhea is seen in patients with secondary amyloidosis. Keywords: Amyloidosis, Colitis, Colonoscopy, Diarrhea |
|
INTRODUCTION
|
|
Amyloidosis may involve the gastrointestinal tract, mainly the upper intestinal tract but involvement of the colon may occur. Gastrointestinal amyloidosis is relatively rare in western countries occurring in 3–8% of patients with systemic amyloidosis, but a recent report from Korea found 15.5% gastrointestinal involvement [1]. The most frequent clinical manifestation in all patients with amyloidosis are weight loss and gastrointestinal bleeding [2]. However, diarrhea is seen in patients with secondary amyloidosis [1]. The majority of patients with amyloidosis (80%) have primary amyloidosis, and most of these patients are over 50 years old [2]. In primary amyloidosis, amyloid deposition occur in the muscularis mucosae, submucosa, and muscularis propria leading to the thickening of intestinal folds, and usually presents with constipation, mechanical obstruction, or chronic intestinal pseudo-obstruction [3]. We present the case of an elderly male with chronic diarrhea and weight loss. Colonoscopy revealed pancolitis and colon biopsy revealed amyloid deposition. |
|
CASE REPORT
|
|
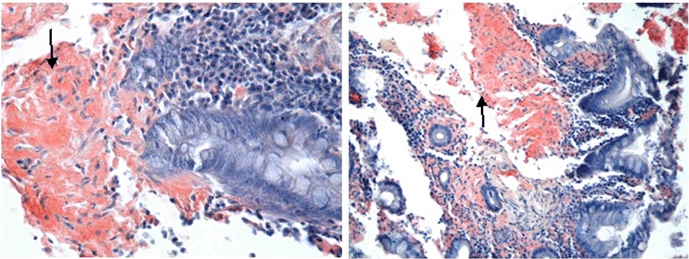
A 76-year-old Jamaican male presented to the University Hospital of the West Indies, Jamaica with a six-month history of diarrhea, which he describes as four episodes of loose non-bloody stools daily. This was associated with anorexia and 10 pounds weight loss in six months. He presented to hospital after experiencing three episodes of bloody diarrhea over a two-day period. He denied urgency, tenesmus, abdominal pain, nausea, vomiting or symptoms of anemia. He has not experienced similar symptoms in the past. He is an ex-smoker and denies any history of recent antibiotic exposure, recent travel or a personal or family history of colon cancer or inflammatory bowel disease. His past medical history was significant for congestive cardiac failure which was thought to be due to ischemic heart disease diagnosed four weeks earlier after presenting with a history of bilateral lower limb edema. The echocardiogram revealed moderate concentric left ventricular hypertrophy with ejection fraction 65–70%, mild diastolic dysfunction, moderately enlarged right atrium, moderately dilated left atrium and small generalized pericardial effusion with no evidence of cardiac tamponade. On examination he was noted to have pale mucous membranes, moderate generalized wasting and moderate pedal edema. His cardiovascular examination was significant for low volume pulses but was otherwise normal. His abdominal examination revealed shifting dullness but no organomegaly. His rectal examination showed a moderately enlarged firm prostate with no focal lesion. His respiratory and nervous system examinations were unremarkable Investigations showed hemoglobin 8.9 g/dl (normal range: 11.5–16.5 g/dl), MCV 83.9 fL (normal range: 81.1–96.0 fL), MCH 27.6 pg (normal range: 27.0–31.2 pg), platelets 229x109/L (normal range: 150–450 x109/L), white blood cell count 5.2x109/L (normal range: 3.75–11.0 x109/L), total protein 37 g/L (normal range: 68–84 g/L), albumin 19 g/L (normal range: 38–52 g/L), globulin 18 g/L (normal range: 18–38 g/L), alkaline phosphatase 240 U/L (normal range: 15–105 U/L), AST 16 U/L (normal range: 7–32 U/L), total bilirubin 11 umol/L (normal range: 4–18 umol/L), ferritin 273 ng/ml (normal range: 16–294 ng/ml), vitamin B12 506 pmol/L (normal range: 156–698 pmol/L), folic acid 6 pg/ml (normal range: 2–20 pg/ml), PSA 3.74 ug/L (normal range: 0.00–4.00 ug/L). His spot urine protein/creatinine ratio was 1.67 g/day. He had normal clotting indices, urea, creatinine and electrolytes. The abdominal ultrasound revealed prostatomegaly with features of chronic bladder outlet obstruction, features suggestive of bilateral renal parenchymal disease, small volume ascites and small bilateral pleural effusions. Colonoscopy showed mild to moderate pancolitis characterized by granularity of the mucosa, blunting of the normal vascular pattern, friability of the mucosa with scattered superficial ulcerations. This was thought to be suggestive of ulcerative colitis. Biopsies, however, revealed colonic mucosa in which the general architecture is distorted by the deposition in the lamina propria of amorphous eosinophilic material. This stained positively with Congo red stains, confirming the presence of amyloid (Figure 1). He was assessed as having colonic amyloidosis as part of secondary amyloidosis. After his presumptive diagnosis of ulcerative colitis he was started on prednisone 40 mg daily with complete resolution of his diarrhea after four days but he continued to experience persistent lower limb edema, ascites and hypoalbuminemia. He was treated symptomatically but he demised in hospital. |
|
|
|
DISCUSSION
|
|
Amyloidosis is a rare disorder and refers to the extracellular deposition of insoluble amyloid fibrils in the tissues of the body leading to end organ damage. Depending on the precursor protein, clinical manifestations may vary significantly. Amyloid can be confined to an organ or may be systemic. In systemic amyloidosis, the heart, kidneys, and nerves are most commonly affected, resulting in congestive heart failure, arrhythmia, nephrotic syndrome, renal failure, and peripheral and autonomic neuropathies [4]. Isolated gastrointestinal amyloidosis is rare [5]. In one series, the most common presentations for gastrointestinal amyloidosis were weight loss (45%) and gastrointestinal bleeding (36%) [5]. In another report, the most common symptoms of gastrointestinal amyloidosis were diarrhea (45.8%), anorexia (37.5%), weight loss and nausea and/or vomiting (29.2%) and the histologically confirmed gastrointestinal tract site was the stomach (55.0%), colon (45.0%) and rectum (35.0%). Patients with gastrointestinal involvement had a greater frequency of organ involvement [1]. The endoscopic appearance of gastrointestinal amyloidosis is non-specific and may include a fine granular appearance of the mucosa, erosions, ulcerations, mucosal friability and polypoid protrusions [6]. These findings may reflect amyloid deposition in the mucosa or submucosa. In one study, the degree of amyloid deposition was most marked in the duodenum and significantly correlated with the frequency of endoscopic findings of fine granular appearance and polypoid protrusions [6]. In secondary amyloidosis (serum acute phase-reactant, amyloid A protein), endoscopic findings have been related to deposition occurring mainly in the propria mucosae with symptoms of diarrhea, as in our patient [3]. The endoscopic findings in our patient were similar to ulcerative colitis. There are no pathognomonic radiologic or endoscopic findings, and diagnosis is usually delayed. Ultimately, a biopsy of the affected region of the gastrointestinal system is required to confirm the diagnosis and exclude other causes. Characteristically, tissue biopsy has positive staining of amyloid by Congo red or amyloid fibrils on electron microscopy. Further evaluation should thereafter be performed to determine the type of amyloid and the underlying cause. Our patient had biopsy proven colonic amyloidosis but in light of his non-nephrotic range proteinuria and cardiomyopathy, he likely had systemic amyloidosis, affecting the heart and kidney in addition to colonic involvement. The intestinal presentation may be similar to several conditions. As a result of the protean manifestations of gastrointestinal amyloidosis, the diagnosis is often delayed and requires a thorough history, physical examination and appropriate investigations including an upper endoscopy and/or colonoscopy. A high index of suspicion should be maintained in any patient presenting with diarrhea and edema so as not to delay diagnosis and treatment Current therapies suppress or stabilize the precursor protein formation and interfere with fibrillogenesis [4]. The treatment of gastrointestinal amyloidosis is aimed at symptomatic control. Patients with severe hypoalbuminemia and chronic diarrhea due to protein-losing enteropathy may respond to combination therapy with a somatostatin analogue, like octreotide and corticosteroid [7][8]. This therapeutic option should be considered not only in AA amyloidosis, but also in systemic amyloidosis, because of the lack of specific therapies in this serious condition [7]. The underlying cause of the amyloidosis should be treated and this may result in lasting regression of the gastrointestinal amyloidosis [9][10][11]. The prognosis of patients with AL amyloidosis (amyloid light-chain amyloidosis) and gastrointestinal involvement was poorer than those without gastrointestinal involvement, and they presented with more organ involvement and more advanced disease than those without organ involvement [1]. |
|
CONCLUSION
|
|
The intestinal presentation may be similar to several conditions as a result of the protean manifestations of gastrointestinal amyloidosis. A high index of suspicion should be maintained in any patient presenting with chronic diarrhea and edema to avoid delay in diagnosis and treatment. |
|
REFERENCES
|
|
|
[HTML Abstract]
[PDF Full Text]
|
|
Author Contributions
Yvonne M. Dawkins – Substantial contributions to conception and design, Acquisition of data, Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published Barrie Hanchard – Substantial contributions to conception and design, Acquisition of data, Revising it critically for important intellectual content, Final approval of the version to be published Michael G. Lee – Substantial contributions to conception and design, Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published |
|
Guarantor
The corresponding author is the guarantor of submission. |
|
Source of support
None |
|
Conflict of interest
Authors declare no conflict of interest. |
|
Copyright
© 2017 Yvonne M. Dawkins et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information. |
|
|