|

|
 |
|
Case Report
| ||||||
| Remarkable body weight gain in a patient in the terminal phase of gastric carcinoma secondary to ‘big IGF-II’— producing tumor | ||||||
| Genta Ishikawa1, Naoki Nishimura1, Teruhiko Yoshida1, Atsushi Kitamura1, Yasuhiko Yamano1, Yutaka Tomishima1, Torahiko Jinta1, Koyu Suzuki2, Naohiko Chohnabayashi2 | ||||||
|
1Division of Pulmonary Medicine, St. Luke’s International Hospital.
2Department of Pathology, St. Luke’s International Hospital. | ||||||
| ||||||
|
[HTML Abstract]
[PDF Full Text]
[Print This Article]
[Similar article in Pumed] [Similar article in Google Scholar] 
|
| How to cite this article: |
| Ishikawa G, Nishimura N, Yoshida T, Kitamura A, Yamano Y, Tomishima Y, Jinta T, Suzuki K, Chohnabayashi N. Remarkable body weight gain in a patient in the terminal phase of gastric carcinoma secondary to ‘big IGF-II’— producing tumor. International Journal of Case Reports and Images 2013;4(7):380–383. |
|
Abstract
|
|
Introduction:
Weight loss as a result of cachexia with malignancy is frequently encountered in clinical practice. However, previous reports have not demonstrated weight gain in terminal phase of malignancy.
Case Report: We report a 69-year-old male admitted with an exacerbation of interstitial pneumonia and multiple metastases of relapsed gastric adenocarcinoma complicated with refractory hypoglycemia. He had gained approximately 10 kg weight with an acromegaloid appearance in a one-month period before admission. The presence of big insulin-like growth fact II (Big IGF-II) in his blood and an immunohistochemical study on the tumor cells confirmed a diagnosis of non-islet cell tumor hypoglycemia. Conclusion: This report possibly illustrates an unreported differential diagnosis of weight gain in terminal phase of malignancy. | |
|
Keywords:
Gastric carcinoma, Non-islet cell tumor hypoglycemia (NICTH), Body weight gain
| |
|
Introduction
| ||||||
|
Non-islet cell tumor hypoglycemia (NICTH) is a rare condition of malignancy. It has been considered as a cause of refractory hypoglycemia, which results from an abnormal secretion of big insulin growth factor-II (Big IGF-II) from tumor cells. This case report describes significant weight gain with an acromegaloid appearance in a 69-year-old male with NICTH associated with Big IGF-II producing tumor in the terminal phase of gastric adenocarcinoma. | ||||||
|
Case Report
| ||||||
|
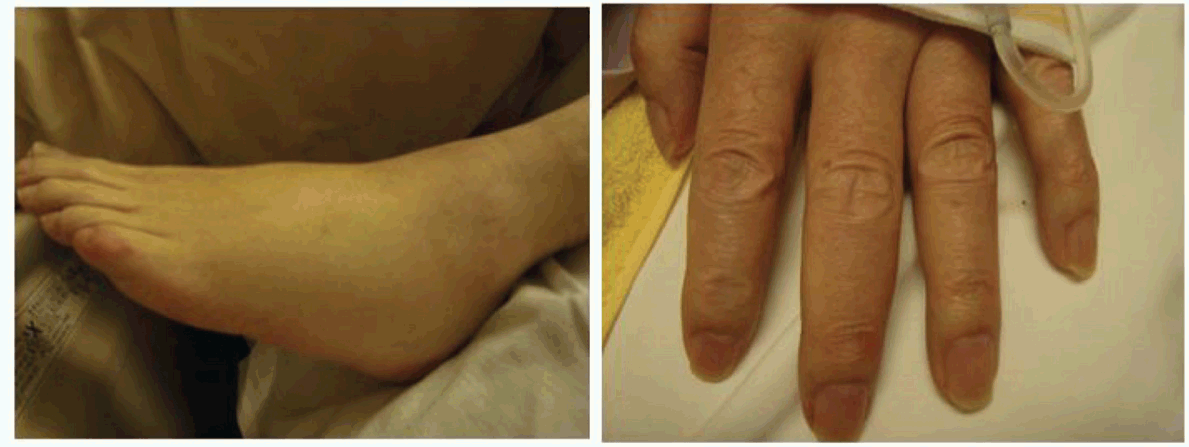
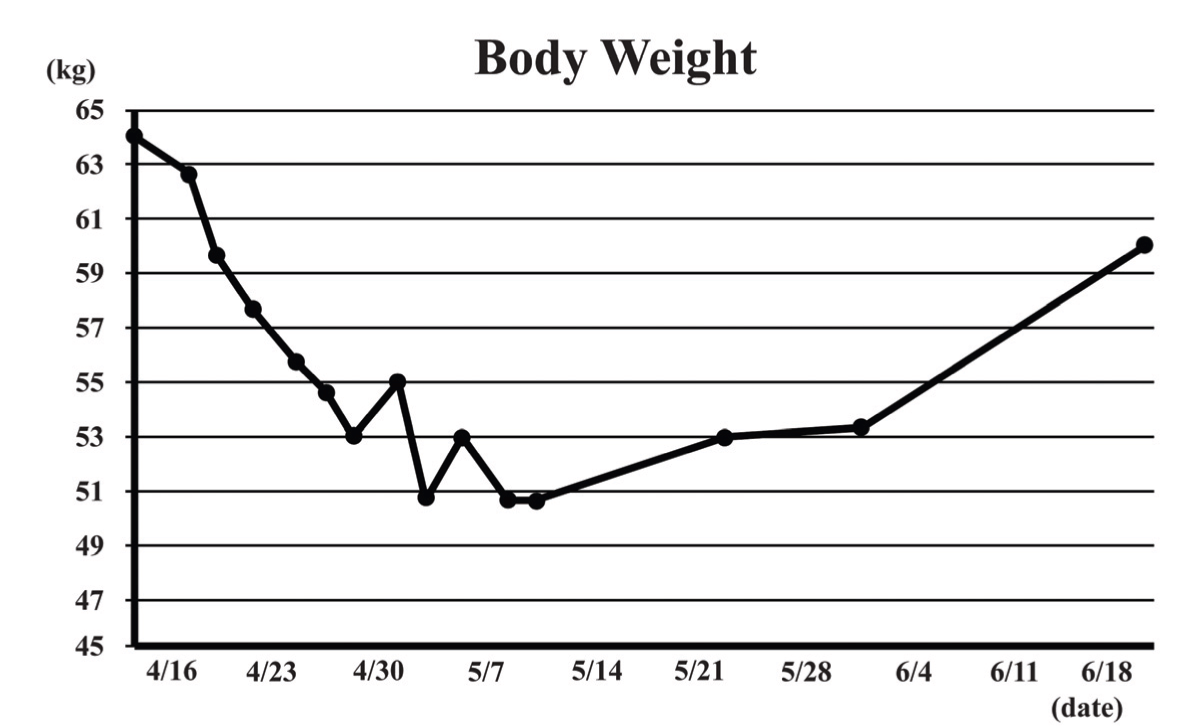
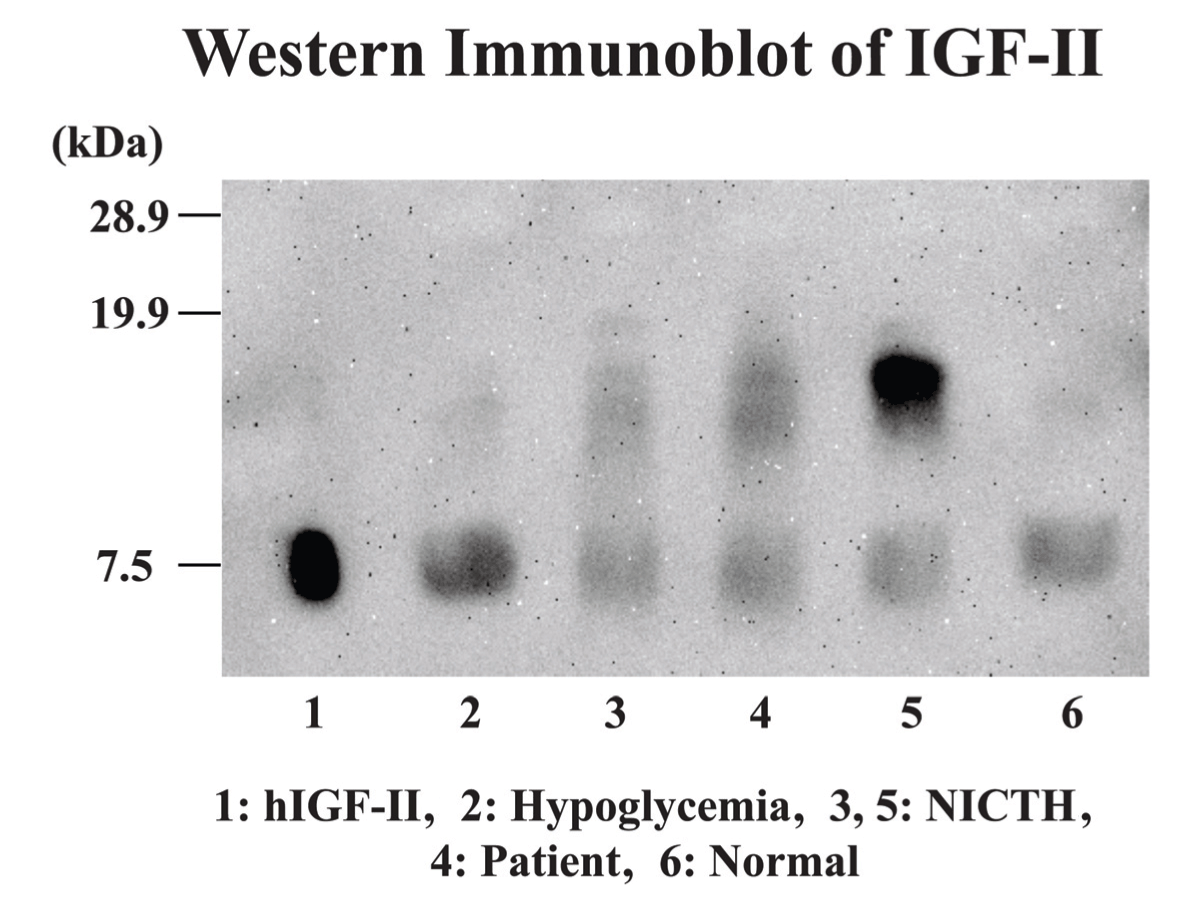
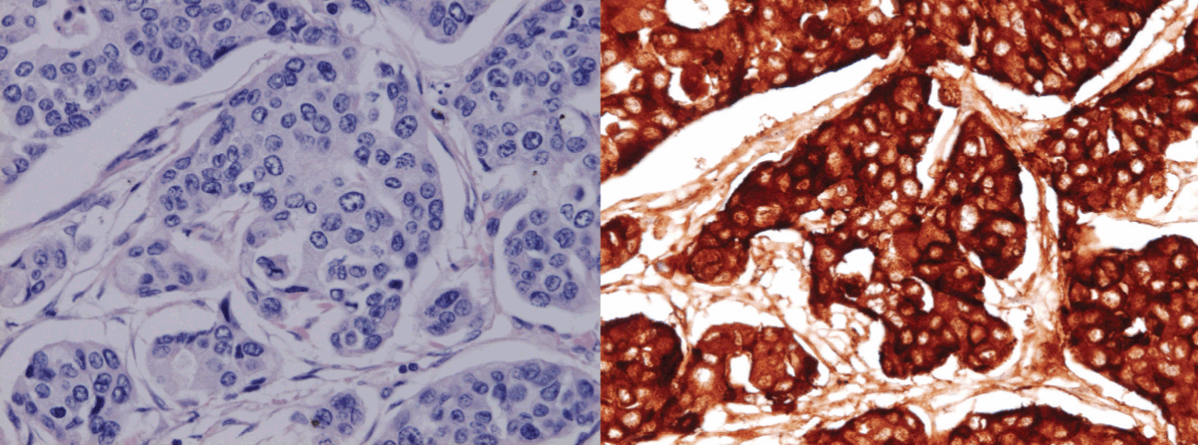
A 69-year-old male with relapsed gastric adenocarcinoma (total gastrectomy and Roux-en-Y reconstruction were performed in September 2009) and systemic metastases was admitted to our hospital for severe respiratory failure as a result of an acute exacerbation of interstitial pneumonia on June 22, 2011. He had gained approximately 10 kg weight with acromegaloid features in a one-month period before his admission. (Figure 1) (Figure 2) He had noticed his shoes were too small for his feet and his wedding ring no longer fit in his finger. On the day of admission, he suffered from severe hypoglycemia (blood sugar 28 mg/dL). He had an acromegaloid appearance with enlargement of hands, prognathism, and increased heel pad thickness without edema in his extremities. (Figure 1) Skin tags, excessive oiliness of the skin and rhinophyma were also observed. He was given 10% dextrose intravenously and 1 mg intramuscular glucagon. In addition, methyl-prednisone 1 g/day was commenced for the exacerbation of interstitial pneumonia. Despite these therapies, his hypoglycemia persisted and 10% dextrose infusion continued to be necessary to maintain the glucose level until his death. The laboratory examination showed no increased levels of insulin (4.0 μU/mL, normal limit 1.7–10.4 μU/mL), growth hormone (1.786 ng/mL, normal limit <5.0 ng/mL) and insulin growth factor-I (IGF-I, 41.8 ng/mL, normal limit 42–250 ng/mL). Furthermore, C-peptide level in the urine sample was suppressed (0.3 μg/day, normal limit 20.1–155.0 μg/day). Western blot analysis of IGF-II in sera obtained at hypoglycemia disclosed a two-fold higher molecular IGF-II (Big IGF-II) when compared with normal human IGF-II (7.5 kDa) (Figure 3). The computed tomography (CT) scan showed diffuse ground glass opacities with traction bronchiectasis throughout the lungs as well as multiple lung metastases with bilateral pleural effusion and mediastinal lymphadenopathy and the metastasis had markedly increased in extent compared to the CT scan performed two months before admission. (Figure 4) Echocardiography demonstrated normal left ventricular contraction, and serum NT-pro-BNP level was 383.4 pg/mL, (normal limit <125.0 pg/mL). Massive proteinuria and severe liver dysfunction had not been observed. An increased thickness of abdominal subcutaneous tissue was detected in CT scan. (Figure 4) The patient died three days after admission due to uncorrectable respiratory failure induced by interstitial pneumonia. The postmortem examination confirmed a diagnosis of poorly differentiated gastric adenocarcinoma with metastases to lungs, thyroid, pancreas and lymph nodes. Diffuse alveolar damage and chronic interstitial pneumonia were also recognized. Immunohistochemical study using polyclonal anti-IGF-II antibody (ab9574; Abcam, Cambridge, UK) demonstrated a positive staining of IGF-II in tumor cells. (Figure 5) Additionally, the immunohistochemistry with anti-IGF-II demonstrated a positive staining in the specimen of primary gastric cancer resected in 2009. Taking into account the detection of Big IGF-II in his blood, the result of immunohistochemistry and refractory hypoglycemia, we made a final diagnosis of NICTH. | ||||||
| ||||||
| ||||||
| ||||||
|
| ||||||
| ||||||
|
Discussion
| ||||||
|
The NICTH is a rare condition and was first reported in 1929 with hepatocellular carcinoma. In 1988, the relationship between the aberrant production of pro-IGF-II (Big-IGF-II) and tumor-induced hypoglycemia was first proposed. Several case reports of tumor-induced hypoglycemia have been published and various tumors can result in NICTH. Data on the exact incidence and prevalence of NICTH are not available. However, it usually occurs in a subject with solid tumors of well-differentiated mesenchymal origin. [1] [2] [3] Also, gastric carcinoma is one of the most possible causes of NICTH, [4] and several case reports have been published. [5] While the synthesis of IGF-I in liver is largely regulated by growth hormone (GH), the synthesis of IGF-II is independent of GH action. IGF-II originally has a role in fetal development, cell proliferation and apoptosis. [6] [7] Both IGF-I and IGF-II are structurally and functionally associated with insulin. Big IGF-II is an immature form of IGF-II, whose bioavailability is markedly increased because of its tendency to cross the capillary barrier and activate the insulin receptors more easily than mature IGF-II. [8] In NICTH patients, the tumor cells overexpress the IGF-II gene and the abnormal IGF-II produced by the tumor causes refractory hypoglycemia by direct interaction with insulin receptors. [9] [10] In addition, IGF-II is a potent mitogen for many cell types in the autocrine, paracrine and endocrine fashions, which possibly indicates that overproduction of Big-IGF-II from residual tumor cells may facilitate tumor growth (anabolic activity). Acromegaloid skin changes, such as skin tags, excessive oiliness of the skin and rhinophyma, have been reported in patients with NICTH. [11] [12] This patient demonstrated the acromegaloid features. Furthermore, this report showed a remarkable finding of weight change during the clinical course. At first, he lost about 10 kg in one month, followed by a gain of approximately 10 kg in the one month before his death. (Figure 2) In radiographic investigation (CT scan), thickened subcutaneous tissue compared to the image taken two months before was observed. We suppose at first the initial body weight loss resulted from cachexia state surpassing anabolic activity with Big IGF-II production. Furhter, we suppose anabolic activity of Big IGF-II towards not only tumor cells but also normal cells possibly accounted for the remarkable weight gain. We ruled out the existence of insulinoma and primary acromegaly with laboratory data. Worsening fluid accumulation was excluded by the physical examinations, unchanged BNP level in his blood and normal ventricular contraction on echocardiography. Obesity was not probable because his diet had not changed during the clinical course. The limitation of our report was we could not investigate thyroid function that could influence weight change. Further, we could not demonstrate histological finding of anabolic state in subcutaneous tissue. | ||||||
|
Conclusion
| ||||||
|
This is the first report demonstrating weight gain in the terminal phase of malignancy. The possibility of Non-islet cell tumor hypoglycemia might be investigated when encountering malignancy with remarkable weight gain. | ||||||
|
Acknowledgements
| ||||||
|
We thank Izumi Fukuda (Department of Medicine, Institute of Clinical Endocrinology Tokyo Women's Medical University) for processing the western blot analysis, and Eri Ishikawa, Miyako Uetake for processing the immunohistochemical study. | ||||||
|
References
| ||||||
| ||||||
|
[HTML Abstract]
[PDF Full Text]
|
|
Author Contributions
Genta Ishikawa – Conception and design, Acquisition of data, Analysis and Interpretation of data, Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published Naoki Nishimura – Conception and design, Acquisition of data, Analysis and Interpretation of data, Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published Teruhiko Yoshida – Conception and design, Acquisition of data, Analysis and Interpretation of data, Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published Atsushi Kitamura – Conception and design, Acquisition of data, Revising it critically for important intellectual content, Final approval of the version to be published Yasuhiko Yamano – Conception and design, Acquisition of data, Revising it critically for important intellectual content, Final approval of the version to be published Yutaka Tomishima – Conception and design, Acquisition of data, Revising it critically for important intellectual content, Final approval of the version to be published Torahiko Jinta – Conception and design, Acquisition of data, Revising it critically for important intellectual content, Final approval of the version to be published Koyu Suzuki- Conception and design, Acquisition of data, Revising it critically for important intellectual content, Final approval of the version to be published Naohiko Chohnabayashi – Conception and design, Acquisition of data, Revising it critically for important intellectual content, Final approval of the version to be published |
|
Guarantor of submission
The corresponding author is the guarantor of submission. |
|
Source of support
None |
|
Conflict of interest
Authors declare no conflict of interest. |
|
Copyright
© Genta Ishikawa et al. 2013; This article is distributed the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any means provided the original authors and original publisher are properly credited. (Please see Copyright Policy for more information.) |
|
|