|

|
 |
|
Case Report
| ||||||
| Intra-aortic metastases or intra-arterial thrombus? | ||||||
| Anna J Lomax1, Saw Yee Yap1, Mustafa Khasraw2 | ||||||
|
1Oncology registrar Andrew Love Cancer Centre Geelong Hospital, Geelong VIC 3220, Australia.
2Oncologist, Andrew Love Cancer Centre, Geelong Hospital, Geelong VIC 3220, Australia. | ||||||
| ||||||
|
[HTML Abstract]
[PDF Full Text]
[Print This Article]
[Similar article in Pumed] [Similar article in Google Scholar] 
|
| How to cite this article: |
| Lomax AJ, Yap SY, Khasraw M. Intra-aortic metastases or intra-arterial thrombus? International Journal of Case Reports and Images 2013;4(7):368–371. |
|
Abstract
|
|
Introduction:
Intra-arterial malignancies are difficult to diagnose as they mimic other more common pathologies such as pulmonary embolus and vascular occlusive disease. Appropriate treatment may, therefore, be delayed.
Case Report: We report a case of an occluded carotid artery and aortic mucosal thickening with a peduculated thrombus occurring in a patient with metastatic sarcomatoid carcinoma while anticoagulated. Conclusion: Diagnosis requires various imaging modalities and a consideration of an alternative diagnosis if not responding to initial therapy. | |
|
Keywords:
Intravascular metastases, Intra-aortic metastases, Arterial thrombus, Sarcomatoid carcinoma
| |
|
Introduction
| ||||||
|
Arterial thrombosis caused by malignancy is less well described in literature compared to venous thromboembolism, which is a well recognized complication of cancer. A thrombotic phenomenon could also be the first manifestation of cancer. We report a patient with metastatic sarcomatoid carcinoma presenting with asymptomatic arterial thrombosis in his common carotid artery and aortic arch. The differential diagnosis included a spontaneous arterial thrombosis due to hypercoagulability, direct tumor invasion of arteries, fragmentation and embolization of intracardiac of tumor. | ||||||
|
Case Report
| ||||||
|
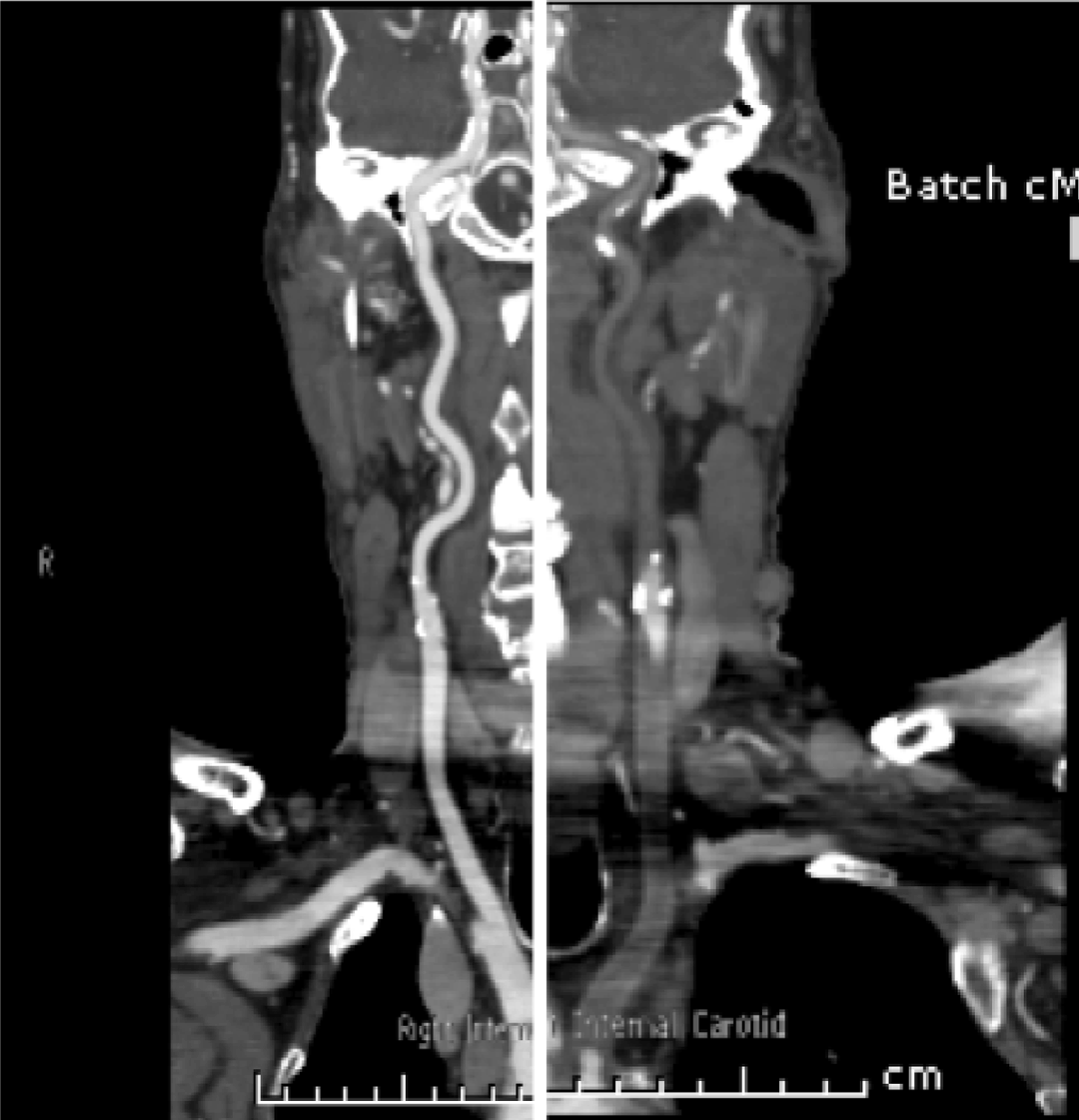
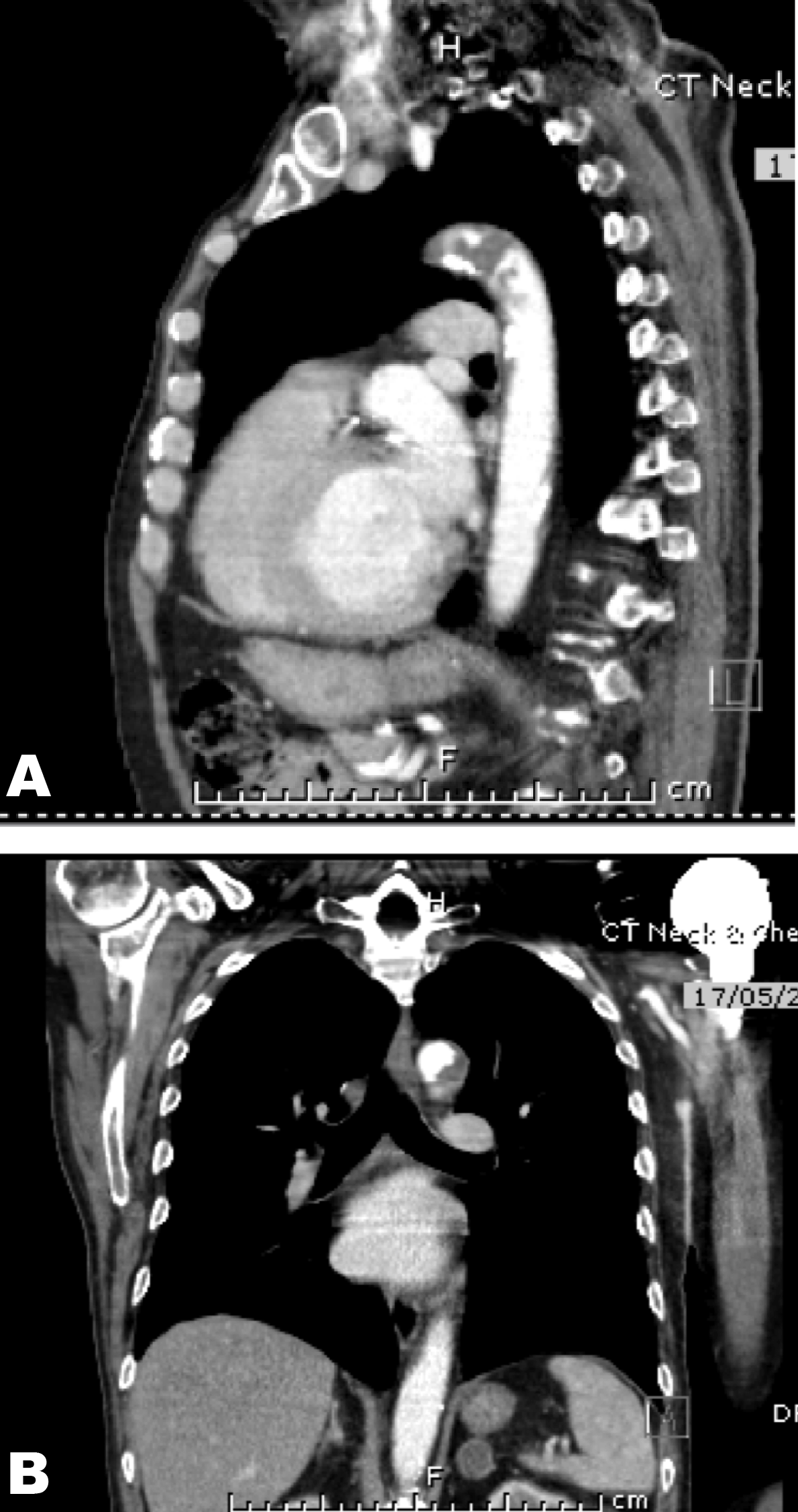
A 73-year-old male with metastatic sarcomatoid carcinoma underwent a restaging computed tomography (CT) scan that incidentally identified complete occlusion of the left common carotid at its origin and narrowing of the origin of the right brachiocephalic artery. (Figure 1) Mucosal thickening was noted with dissection through the mucosa and atheromatous plaques in the aortic arch with a pedunculated 1.9 cm thrombus. (Figure 2 A–B) The lesion was suspicious for metastasis. This was in the setting of a supratherapeutic INR of 3.2 while anticoagulated with warfarin for atrial fibrillation. The left common carotid artery was patent on imaging performed two months earlier. A positron emission tomography (PET) scan confirmed increased metabolic activity in the adrenals and left 7th rib but not in the aorta or left carotid. Magnetic resonance imaging (MRI) scan did not identify intracranial tumor. Five months before, the patient underwent a resection and replacement of his proximal left humerus due to malignant infiltration. Histopathology revealed features of a malignant spindle cell neoplasm favoring metastatic sarcomatoid carcinoma rather than bone primary. However, the primary site was unknown. He was initially managed with postoperative radiotherapy to the surgical bed (20 Gy, 5 fractions) plus a boost to the scar with a plan to follow with serial imaging. Other past history included significant peripheral vascular disease and diabetes. He was commenced on palliative intent chemotherapy with carboplatin and gemcitabine after the restaging scan. Warfarin was ceased and enoxaparin commenced. Left carotid embolectomy was deemed to be too risky. Prophylactic intervention to the right carotid was considered appropriate only if the patient were to have a good response to chemotherapy or became symptomatic of the stenosis. | ||||||
| ||||||
| ||||||
|
Discussion
| ||||||
|
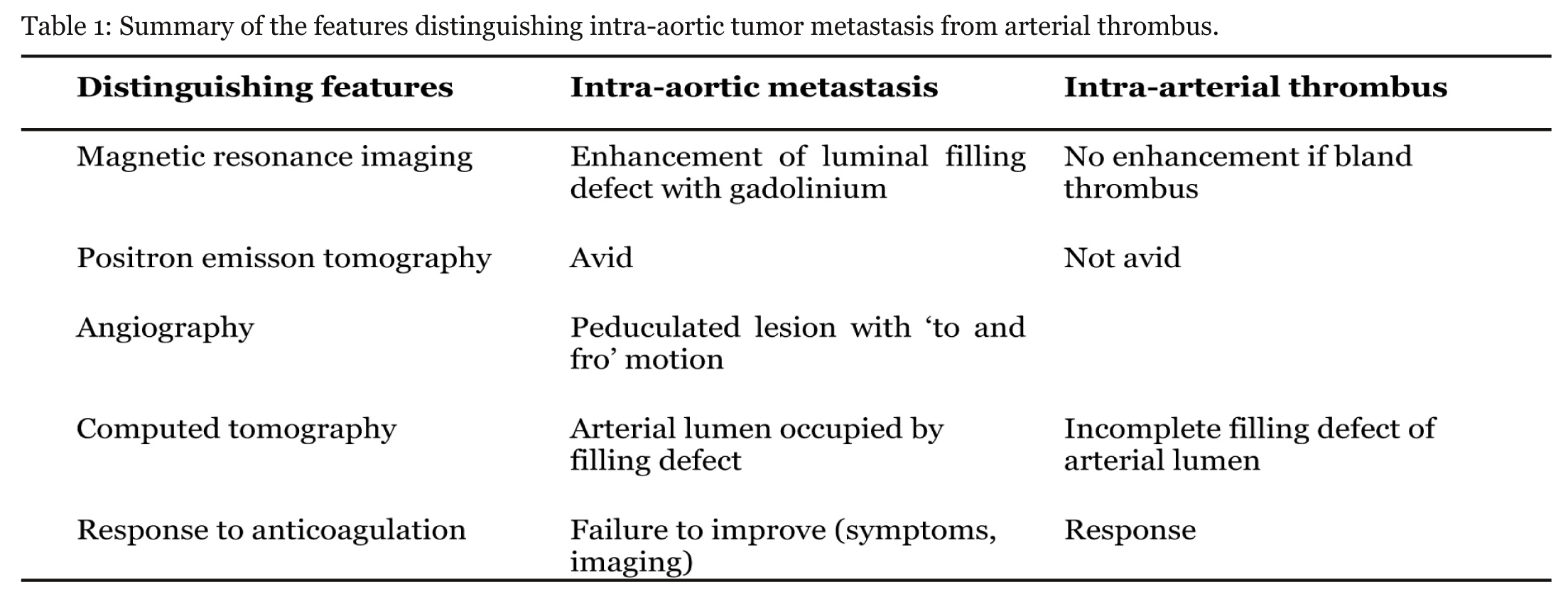
Sarcomatoid carcinomas are uncommon tumors that are not specific to a particular anatomical site of origin. Features representative of carcinoma and sarcoma are present in variable proportions.[1] The carcinoma component consists of malignant epithelial cells whereas the sarcomatoid appearance is due to mesenchyme-like cells. This may occur through phenotypic conversion of carcinoma to sarcoma resulting in a more biphasic or monophasic tumor. [1] Those arising in a hollow organ may be pedunculated in appearance but can also have ulcerative or infiltrative chracteristics. [1] Carotid, aortic and pulmonary artery sarcomas (PAS) mimicking other pathologies such as vascular occlusive disease, aneurysms and pulmonary emboli are described in case reports. [2] [3] [4] [5] [6] [7] [8] [9] [10] [11] [12] Our patient represents a similar clinical scenario. The principles from these cases could be applied here. Sarcomas of the aorta are rare and tend to involve the thoracic and abdominal aorta. [11] They may be intimal or mural. The former are more aggressive with plaque formation along the aorta or polypoid growth into the lumen, causing vessel occlusion and distal embolization. Mural sarcomas are less aggressive with invasion of surrounding structures. [3] [6][11] A paper examining the CT findings of pulmonary artery sarcoma and pulmonary emboli found some distinguishing features. [13] In PAS, a low-attenuation filling defect that occupies the entire luminal diameter of the main or proximal pulmonary artery was seen. There may be arterial expansion of the affected artery. Extra-luminal tumor extension may be present. Less common features include heterogenous enhancement and unilateral distribution. An MRI with gadolinium-diethylenetriamine may aid diagnosis with enhancement of the filling defect indicating intraluminal tumor. This would not occur if bland non-vascularized thrombus were present. [2] [10] [13] The 18-fluoro-deoxyglucose (FDG)–PET/CT positive uptake has been demonstrated within a pulmonary artery sarcoma further aiding diagnosis and excluding bland thrombus. [2] [4] [14] The lack of PET enhancement in this case may be due to low volume of disease. Other factors supporting the diagnosis include a pedunculated or polypoid lesion with to and fro motion on angiography. [7] [10] This may not be apparent if there is a sessile tumor or excessive thrombus over the tumor. [2] Diagnosis may occur after failure of improvement in symptoms or a lack of response to anticoagulation on imaging. [10] [12]Diagnosis may be incidental after surgical intervention such as pulmonary thrombolectomy [10] and aneurysm repair. [6] The management of malignant intra-arterial disease involves a combination of resection or debulking, chemotherapy and radiotherapy. [7] [14] Table 1 summarizes the features distinguishing intra-aortic metastasis from intra-arterial thrombus. | ||||||
| ||||||
|
| ||||||
|
Conclusion
| ||||||
|
In summary, arterial vascular occlusion may be due to thrombosis or less commonly, direct tumor involvement requiring different medical management. Arterial malignancies are difficult to diagnose. Multimodality imaging such as computed tomography scan, magnetic resonance imaging scan, positron emission tomography is required and each provides additional information differentiating arterial malignancy from pure thrombus. Clinical factors, such as thrombus not responding to adequate anticoagulation and an index of suspicion, may prompt further investigation. | ||||||
|
References
| ||||||
| ||||||
|
[HTML Abstract]
[PDF Full Text]
|
|
Author Contributions
Anna J Lomax – Substantial contributions to conception and design, Drafting the article, Final approval of the version to be published Saw Yee Yap – Acquisition of CT images, Drafting the introduction, Final approval of the version to be published Mustafa Khasraw – Contributions to conception and editing of article, Drafting the article, Final approval of the version to be published |
|
Guarantor of submission
The corresponding author is the guarantor of submission. |
|
Source of support
None |
|
Conflict of interest
Authors declare no conflict of interest. |
|
Copyright
© Anna Lomax et al. 2013; This article is distributed the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any means provided the original authors and original publisher are properly credited. (Please see Copyright Policy for more information.) |
|
|