| Table of Contents |  |
|
Case Report
|
| Is sodium thiosulfate a therapeutic option for non-uremic calciphylaxis? |
| Ashish Bhargava1, Vasavi Paidpally2, Pragati Bhargava2, Alexander Vonbank1,2,3 Christoph Mayr1 Edgar Meusburger1 Karl Lhotta1,2 |
|
1Department of Nephrology and Dialysis, Academic Teaching Hospital Feldkirch, Austria.
2Vorarlberg Institute for Vascular Investigation and Treatment (VIVIT), Feldkirch, Austria. 3Private University of the Principality of Liechtenstein, Triesen, Liechtenstein. |
|
doi:10.5348/ijcri-2012-08-161-CR-8
|
|
Address correspondence to: Alexander Vonbank Department of Nephrology and Dialysis, Academic Teaching Hospital Feldkirch Carinagasse 47 A-6800 Feldkirch Austria Phone: 43 5522 303 2670 Fax: +43 5522 303 7506 Email: alexander.vonbank@lkhf.at |
|
[HTML Abstract]
[PDF Full Text]
|
| How to cite this article: |
| Vonbank A, Mayr C, Meusburger E, Lhotta K. Is sodium thiosulfate a therapeutic option for non-uremic calciphylaxis? International Journal of Case Reports and Images 2012;3(8):27–30. |
|
Abstract
|
|
Introduction:
Non-uremic calciphylaxis, a rare disorder with high mortality, is associated with severe calcifications of the media of arterioles which cause vascular occlusion and tissue necrosis.
Case Report: A non-uremic calciphylaxis of the lower limb, possibly caused by coumarin therapy for atrial fibrillation. Treatment consisting of coumarin avoidance, vitamin K supplementation and thrice weekly infusion of 20 g of sodium thiosulfate for three weeks led to complete healing of the leg ulcers. Conclusion: Sodium thiosulfate produces a considerable sodium load and may induce a decrease in serum calcium, potassium and phosphate levels as well as an increase in the anion gap. We suggest that sodium thiosulfate is effective in the treatment of non-uremic calciphylaxis, but its use requires monitoring of electrolytes, acid-base and volume status. Based on previous studies of sodium thiosulfate pharmacokinetics in individuals with normal renal function, which showed rapid excretion by the kidneys, application as a slow infusion over several hours is recommended in order to enhance its efficacy and avoid unwanted side effects. | |
|
Key Words:
Calciphylaxis, Sodium thiosulfate, Pharmacokinetics
| |
|
Introduction
| ||||||
|
Calcific uremic arteriolopathy (CUA), also called calciphylaxis, is a rare small vessel disease almost exclusively found in patients with end-stage renal disease.[1] Severe calcification of the media of arterioles cause vascular occlusion and tissue necrosis. The pathogenesis of the condition is incompletely understood. It appears to be associated with deranged calcium and phosphate metabolism and a lack of inhibitors of calcification secondary to chronic kidney disease. [2] Compared to CUA, coumarin necrosis is associated with protein-c deficiency and local microthromboembolic events. Non-uremic calciphylaxis (NUC) seems to be extremely rare. A recent review of literature described 36 cases of NUC. [3] According to this report, NUC is associated with a high mortality of 52% and an effective treatment for this condition does not exist. [3] A patient with NUC who had a complete remission under sodium thiosulfate (STS) therapy and explain the alterations in serum and urinary electrolytes caused by STS is discussed here. | ||||||
|
Case Report
| ||||||
|
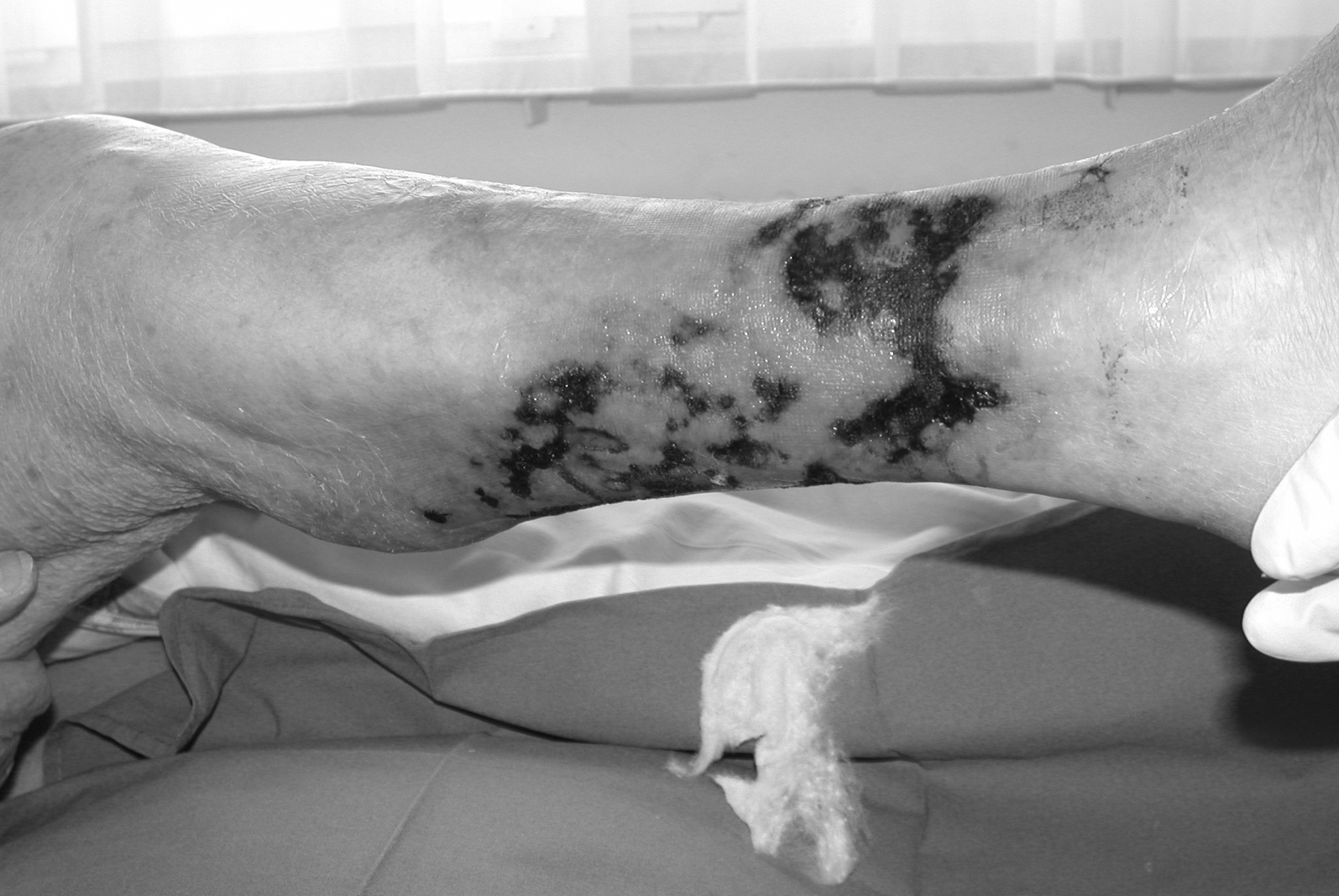
The patient, an 88-year-old Caucasian woman, had a medical history of coronary artery disease, hypertension, ischemic stroke and permanent atrial fibrillation (CHADS2 score 5 points) requiring oral anticoagulation with coumarins (initial INR value of 3.9). The patient was on coumarin therapy for 1.5 years. She presented with a two-week history of extremely painful circular necrotic ulcerations on the right lower leg (Figure 1). Peripheral artery disease was excluded with arterial duplex sonography with a Philipps iU22 ultrasound system. A soft tissue radiogram of the lower right leg using mammography film (low voltage and high resolution) showed small vessel calcification. A skin biopsy revealed extensive tissue necrosis and was otherwise inconclusive. A diagnosis of NUC, possibly caused by coumarin therapy, was made on the basis of these clinical and radiological findings. The patient's condition deteriorated rapidly, with an increase in ulcerations and severe pain necessitating intravenous morphine. At hospital admission, the patient had normal serum calcium levels (2.27 mmol/L), borderline hypophosphatemia (0.77 mmol/L) and an eGFR of 59 mL/min. Parathyroid hormone (PTH) levels were elevated (116 pg/mL), and the patient had low 25-OH vitamin D3 levels (19.4 nmol/L). Coumarin therapy was discontinued and anticoagulation changed to low molecular heparin (enoxaparin). Intensive wound care was performed with chlorhexidine paraffin dressing (Bactigras®) and sulfadiazine unguent. The patient received 20 mg of oral vitamin K per day. Furthermore, intravenous STS 20 g per day, infused over 30 min, three times per week, was started. This infusion therapy was continued over the next three weeks. Therapy was well tolerated. For safety reasons and to determine STS pharmacokinetics, we measured serum and urine electrolytes before and immediately after STS infusion (Table 1). The ulcers on both legs improved rapidly after a three-week treatment. Analgesic therapy was finally reduced to low-dose oral therapy. Two months later, the leg ulcers had completely healed (Figure 2). | ||||||
| ||||||
| ||||||
| ||||||
|
Discussion
| ||||||
|
To our knowledge, there is only one report of successful treatment of NUC with STS. [4] NUC itself seems to be extremely rare. In their review of 36 cases Nigwekar et al. [3] describe several risk factors for the disease, among them female gender, non-uremic chronic kidney disease and coumarin use, all of which were present in our patient. It has been suggested that coumarins can alter the balance of calcification promoters and inhibitors by inhibiting vitamin K-dependent carboxylation of matrix GLA protein, a potent inhibitor of calcification, [5] and in animal models coumarins have been shown to precipitate vessel calcification. [6] In the above case report of a patient with NUC and hypoparathyreoidism, STS had to be administered over a prolonged period of 46 weeks. [4] We observed complete remission of leg ulcers after only three weeks of treatment. This difference may be explained by the far more advanced disease affecting the abdominal wall in the patient described by Hackett et al. [4] Another report describes successful treatment of iatrogenic calcinosis cutis, which was caused by extravasation of calcium gluconate during treatment for tumor lysis syndrome in a child with lymphoblastic leukemia. [7] Cicone et al. [8] reported the first case of successful treatment of calciphylaxis with STS in a patient with chronic renal failure in 2004. The beneficial effect was ascribed to the fact that calcium thiosulfate salts have an extremely high solubility and that STS therefore dissolves already precipitated calcium salts in the vessels. Furthermore, STS has a potent antioxidant effect, which helps neutralize reactive oxygen species, and generates the antioxidant glutathione. [9] In addition, STS is metabolized to hydrogen sulfide, a potent vasodilator. This last effect may explain the rapid improvement in pain associated with STS use. Further case reports and small case series have been documented successful treatment of calciphylaxis with sodium thiosulfate. [10] A 10% STS solution (100 mL) contains 40 mmol thiosulfate (TS) and 80 mval Na+. Thus, each treatment with 20 g STS contained 80 mmol TS and 160 mval Na+. In order to study any other effects of STS on electrolyte metabolism, we measured serum electrolytes immediately before and after infusion of STS. In serum, we detected a small decrease in ionised calcium, possibly caused by its complexation with TS or by an increase in renal calcium loss. This drop in ionised calcium was accompanied by an increase in the PTH level. We also found a decrease in serum potassium despite stable renal potassium excretion. Despite the sodium load, serum sodium remained constant. Another unexpected finding was a marked drop in serum phosphate levels. A possible explanation would be intracellular shifting of anionic phosphate caused by the infusion of TS anion. Previous reports describe a high anion gap acidosis in uremic patients treated with STS. [8] We observed a small decrease in pH, a drop in bicarbonate and an increase in the anion gap. Assuming that this increase of 7 mval represents TS, which is a divalent anion, a serum TS concentration of 3.5 mmol/L can be calculated. In the urine, we observed massive natriuresis accompanied by a more than twofold increase in calcium excretion. Urinary phosphate fell, probably as a consequence of the drop in serum phosphate. We also found a marked increase in the urine anion gap from 60 to 260 mval. We suspect that this unidentified anion is TS at a concentration of 100 mmol/L. From these data, we conclude that a large proportion of the infused STS were rapidly excreted by the kidneys. Under normal conditions only small amounts of TS are produced in the body by metabolism of sulfur containing amino acids. Normal serum levels are around 0.1 mmol/L. [11] The main physiologic function of TS is detoxification of cyanide to thiocyanate by the mitochondrial enzyme rhodanese (thiosulfate cyanide transsulfurase). Thiosulfate is freely filtered in the glomeruli and almost completely reabsorbed by the tubuli. [11] This is mediated by the sodium-coupled transporters NaS1 and NaS2, members of the SLC13 family expressed by proximal tubular cells. [12] However, tubular reabsorption capacity seems to be very limited and infused STS is rapidly excreted by the kidney. Indeed, STS infusion has been used to measure glomerular filtration rate in the past.[13] Studies of a bolus injection of 150 mg/kg STS in healthy volunteers showed a hundred-fold increase in serum TS levels immediately after injection with a rapid fall towards normal levels within three hours. [11] This fall was caused by distribution of TS in the extracellular fluid and renal excretion of 42% of the injected TS in the urine within the three-hour period. In another study, in which 12 g/m2 STS was infused over six hours in patients also receiving cisplatin chemotherapy, only 28% of TS was recovered in the urine up to four hours after infusion. [14] In that study peak TS concentrations in plasma were on average 1.5 mmol/L, which is considerably lower than the level estimated in our patient, and again fell to normal values within three hours. The results of these studies are in agreement with the serum and urine TS levels we derived from measuring the serum and urine anion gaps. These data also suggest that when administered at slower infusion rates more TS is retained in the body than for a bolus infusion, thus inferring that at a slow infusion rate more TS is oxidised to sulfate and metabolised to hydrogen sulfide and thereby exerts an antioxidative and vasodilatory effect. We would therefore suggest that in patients with preserved renal function STS should be infused over a period of several hours to increase its efficacy. Short-time bolus infusion will cause rapid renal excretion of a large proportion of infused TS. Long-term infusion will also avoid high peak concentrations, which cause nausea and vomiting. [11] In dialysis patients, who do not have significant urinary excretion of thiosulfate, STS is expected to remain in the body for a longer period of time and be able to induce its beneficial effects. Whether the amount of TS retained in the body in our patient was high enough to exert any positive effect on NUC is presently unknown. In addition to STS our patient was treated by discontinuing coumarins, prescribing oral vitamin K and local wound care, all of which may have contributed to rapid improvement and remission of the disease. | ||||||
|
Conclusion
| ||||||
|
In conclusion, we describe a patient with NUC, who showed rapid and complete remission following STS infusion therapy. Based on our results and other pharmacokinetic studies we suggest that STS should be given as a slow infusion over several hours rather than as a rapid bolus infusion in order to avoid side effects and increase its effectiveness. Under consideration of our data STS therapy requires careful monitoring of electrolytes and acid-base changes. Attention should also be paid to the high sodium content of STS, which may cause volume overload. Otherwise, STS appears to be a rather non-toxic substance without severe side effects. Nevertheless, more studies on its efficacy and side-effects in NUC are warranted. | ||||||
|
References
| ||||||
| ||||||
|
[HTML Abstract]
[PDF Full Text]
|
|
Author Contributions:
Alexander Vonbank – Substantial contributions to conception and design, Acquisition of data, Analysis and interpretation of data, Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published Christoph Mayr – Substantial contributions to conception and design, Acquisition of data, Analysis and interpretation of data, Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published Edgar Meusburger – Substantial contributions to conception and design, Acquisition of data, Analysis and interpretation of data, Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published Karl Lhotta – Substantial contributions to conception and design, Acquisition of data, Analysis and interpretation of data, Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published |
|
Guarantor of submission:
The corresponding author is the guarantor of submission. |
|
Source of support:
None |
|
Conflict of interest:
Authors declare no conflict of interest. |
|
Copyright:
© Alexander Vonbank et al. 2012; This article is distributed the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any means provided the original authors and original publisher are properly credited. (Please see Copyright Policy for more information.) |
|
|