| Table of Contents |  |
|
Clinical Image
|
| Severe metastatic calcification in patient with transplanted kidney failure |
| Norio Nakamura1, Takeshi Fujita2, Reiichi Murakami2, Ken Okumura3 |
|
1Associate Professor, Community Medicine, Hirosaki University Graduate School of Medicine, Hirosaki-City, Aomori, Japan.
2Assistant Professor, Department of Nephrology, Hirosaki University Graduate School of Medicine, Hirosaki-City, Aomori, Japan. 3Professor, Department of Nephrology, Hirosaki University Graduate School of Medicine, Hirosaki-city, Aomori, Japan. |
|
doi:10.5348/ijcri-2012-07-153-CI-14
|
|
Address correspondence to: Norio Nakamura, MD, Ph.D Hirosaki, Aomori Japan - 0358562 Phone: +81-172-395057 Fax: +81-172-359190 Email: nnakamur@r2.dion.ne.jp |
|
[HTML Abstract]
[PDF Full Text]
|
| How to cite this article: |
| Nakamura N, Fujita T, Murakami R, Okumura K. Severe metastatic calcification in patient with transplanted kidney failure. International Journal of Case Reports and Images 2012;3(7):56–57. |
|
Case Report
| ||||||
|
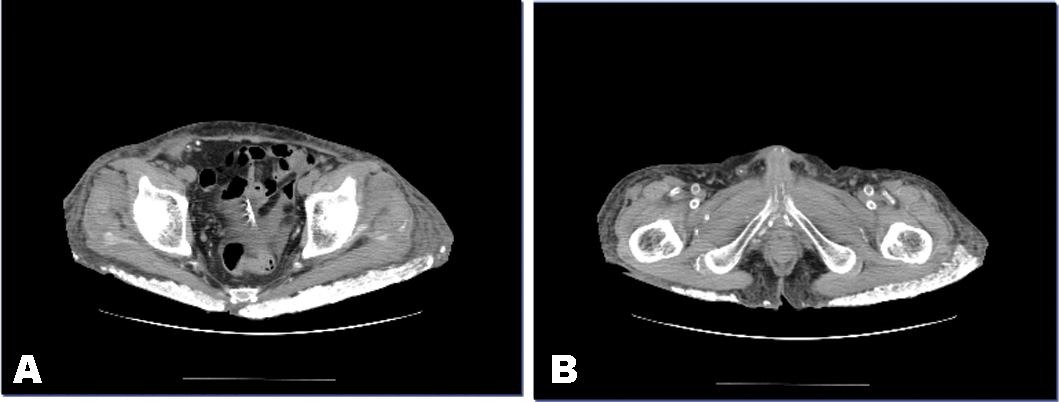
A 45-year-old man with chronic kidney graft failure was admitted to our hospital for dialysis. He had undergone kidney transplantation at 15 years of age because of unspecified glomerulonephritis. After the transplantation, immunosuppressive therapy was administered and the transplanted kidney function was maintained in a good condition. However, the transplanted kidney failure developed gradually, probably because of chronic rejection. The patient showed general fatigue, poor appetite and marked leg edema. He underwent hemodialysis on admission to our hospita. His condition improved gradually. However, he had lumbago and had stiffness of the hip. His abdominal computed tomography (CT) scan is shown in Figure 1. Remarkable metastatic calcification was noted around his hips. His laboratory results were as follow: Hb - 7.1 g/dL, serum total protein - 5.2 g/dL, serum albumin - 2.0 g/dL, BUN - 49 mg/dL, Cr - 6.3 mg/dL, Na - 134 mEq/L, K - 4.3 mEq/L, Cl - 103 mEq/L, Ca - 10.2 mg/dL, P - 6.2 mg/dL, ALP - 488 U/L, serum ß2-microgloburin - 35.9 µg/mL, CRP - 3.8 mg/dL, intact-PTH - 6 pg/mL. Because serum calcium levels were very elevated, the administration of vitamin D3 [1a, 3a-(5Z, 7E)-isomer 1-hydroxycholecalciferol] was discontinued | ||||||
| ||||||
|
Discussion
| ||||||
|
Metastatic calcification is usually observed in patients with end-stage renal disease. [1] Generally, they are observed around a joint, soft tissue, vascular wall, lung, and heart, and they often induce acute arthritis, hypertension, hypoxemia and heart failure. [1] Metastatic calcification is associated with the metabolism of phosphate, calcium and parathyroid hormone. Consequently, for successful treatment, controlling the serum levels of phosphate, calcium and parathyroid hormone is very important. [2] The agents we can use as a drug for metastatic calcification are calcium carbonate, sevelamer and etidronate (bisphosphonate). [3] | ||||||
|
Conclusion
| ||||||
|
Severe metastatic calcification such as that observed in the present case is very rare, however, we should pay attention to serum level of calcium in patients with end-stage renal disease. | ||||||
|
References
| ||||||
| ||||||
|
[HTML Abstract]
[PDF Full Text]
|
|
Author Contributions:
Norio Nakamura - Conception and design, Acquisition of data, Analysis and interpretation of data, Drafting the article, Critical revision of the article, Final approval of the version to be published Takeshi Fujita - Conception and design, Acquisition of data, Analysis and interpretation of data, Drafting the article, Critical revision of the article, Final approval of the version to be published Reiichi Murakami - Conception and design, Acquisition of data, Analysis and interpretation of data, Drafting the article, Critical revision of the article, Final approval of the version to be published Ken Okumura - Conception and design, Acquisition of data, Analysis and interpretation of data, Drafting the article, Critical revision of the article, Final approval of the version to be published |
|
Guarantor of submission:
The corresponding author is the guarantor of submission. |
|
Source of support:
None |
|
Conflict of interest:
Authors declare no conflict of interest. |
|
Copyright:
© Norio Nakamura et al. 2012; This article is distributed the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any means provided the original authors and original publisher are properly credited. (Please see Copyright Policy for more information.) |
|
|