|
Case Report
Capecitabine induced coronary vasospasm
1 St Vincent Hospital, Worcester, USA
2 Lahey Hospital and Medical Center, Burlington, MA, USA
Address correspondence to:
Nitish Kumar Sharma
Saint Vincent Hospital, Department of Medicine, 123, Summer Street, Worcester, MA, 1608,
USA
Message to Corresponding Author
Article ID: 100963Z01NS2018
Access full text article on other devices

Access PDF of article on other devices
pdf.gif)
How to cite this article
Sharma NK, Shah P, Ganatra S, Sharma A. Capecitabine induced coronary vasospasm. Int J Case Rep Images 2018;9:100963Z01NS2018.ABSTRACT
Introduction: Capecitabine is used for malignancies of the breast, stomach, pancreas and hepatobiliary system. Capecitabineinduced cardiotoxicity has been well described. However, there are few studies showing capecitabine induced coronary vasospastic angina. Here, we describe a case of capecitabineinduced coronary vasospasm.
Case Report: A 54-year-old female with a history of metastatic breast cancer on recently started Capecitabine presented with intermittent chest discomfort on exertion. She was ruled out for the acute coronary syndrome. During the hospital stay, the patient had two further episodes of typical chest pain. She then underwent an exercise stress test and was noted to have ST-segment elevation in the inferolateral leads. Given the positive stress test, a coronary angiography was done, that showed no significant obstructive coronary artery disease. Therefore, a diagnosis of Capecitabine induced coronary spasm was made as a diagnosis of exclusion. Capecitabine was stopped and her chest discomfort resolved. The patient was seen as a follow up two months after this episode and she has been chest painfree after the change in her chemotherapy.
Conclusion: Our patient was diagnosed with coronary artery vasospasm secondary to capecitabine and furthermore, discontinuation of capecitabine resolved her symptoms. Recognition of the complication i.e coronary artery vasospasm secondary to capecitabine by the physicians and patients can prevent further adverse events. Also, discontinuation of the drug can further avoid risk for cardiotoxicity.
Keywords: Capecitabine, Chest pain, Coronary angiography, ST-segment elevation, Vasospastic angina
INTRODUCTION
Capecitabine is an oral fluoropyrimidine antimetabolite, which is converted by thymidine phosphorylase to 5-fluorouracil (5-FU). Although it is simply considered an oral version of 5-FU, designed to mimic 5-FU continuous infusion. Capecitabine has the important advantage of being able to concentrate in the targeted tumor [1], resulting in favorable efficacy and toxicity profiles. Moreover, Capecitabine is more convenient for patients, most of whom prefer oral to intravenous treatment, especially in a palliative setting and all these properties justify its increased use [2]. Capecitabine-induced cardiotoxicity is thought to occur through the action of 5-FU on the endothelium resulting in the production of endothelin-1 and subsequent coronary vasospasm. The cardiotoxicity of fluoropyrimidines includes vasospasm, hypertension, ventricular arrhythmias, cardiogenic shock, and even cardiac arrest. The mean reported time interval between 5-FU administration and the onset of cardiac symptoms is three days (range two to five days) [3],[4]. Symptoms are usually relieved within 48 hours after drug discontinuation, but they usually recur when medication is restarted [1].
CASE REPORT
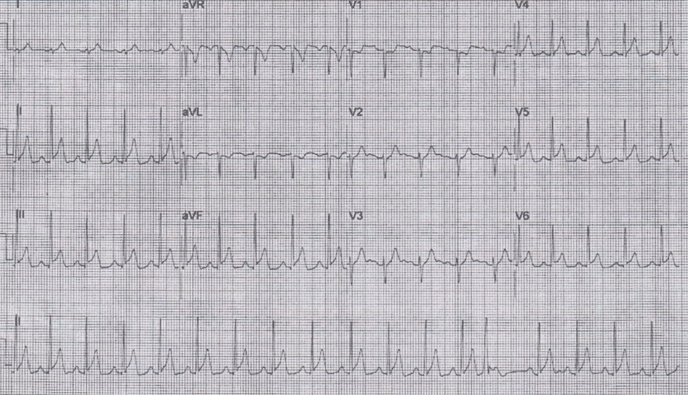
A 54-year-old female with background medical history of metastatic breast cancer status post(s/p) bilateral mastectomy, s/p chemoradiation with relapse presented with intermittent chest discomfort on exertion. She was started on Capecitabine (1250 mg/m2) four days prior to admission. She has initially ruled out for an acute coronary syndrome with negative serial troponins and her resting echocardiogram was normal with no wall motion abnormalities and no valvular pathologies. Her baseline electrocardiogram (ECG) did not have any ischemic ST changes (Figure 1). In the hospital, the patient had two further episodes of typical chest pain but her troponin continued to stay negative with a normal electrocardiogram (EKG). She then underwent an exercise stress test on account of her typical chest pain and after 6 minutes on the treadmill, developed chest discomfort and was noted to have ST elevation in the inferolateral leads consisting of leads II, III, aVF, V5 and V6 (Figure 2). Given the positive stress and a history of radiation to the chest wall, a coronary angiography was considered to evaluate for coronary involvement. Coronary angiography showed no significant obstructive coronary artery disease (Figure 3, Figure 4). The patient did not note any other history of significant medications or drugs or a history of unstable angina in the past. Troponin was checked which was
DISCUSSION
Capecitabine is used for malignancies of the breast, stomach, pancreas and hepatobiliary system. Capecitabine-induced cardiotoxicity has been well described. The most frequent ECG abnormalities associated with capecitabine are acute ST-segment changes and T wave inversion, mostly resolved from within a few hours to up to three days after withdrawal of the drug. Initiation of capecitabine again at the reduced dose has been studied in the retrospective study of 668 patients [5] and a prospective study of 664 patients [6] reported benefit from dose reduction and initiating antianginal drugs at retreatment in 9 of 12 patients and 12 of 15 patients, respectively with strict cardiac monitoring. Moreover, a retrospective study done by Jensen SA et al, symptoms were abolished by nitroglycerine [5]. Prophylactic use of calcium channel blockers failed to demonstrate any effect on the occurrence of cardiotoxicity [7]. Larger studies regarding capecitabine dose reduction and use of antianginal therapy are warranted.
Prior history of ischemic heart disease is the strongest predisposing factor for capecitabine induced cardiotoxicity [8]. A number of studies demonstrated the importance of preexisting cardiac disorders factor for capecitabine induced cardiotoxicity. Some retrospective studies [9] and a prospective cohort study found that patients with the preexisting cardiovascular disease have a significantly increased risk for cardiotoxicity (risk ratio = 6.83) compared to those without baseline cardiac disease [10].
Fluoropyrimidine-induced cardiovascular side effects are expected to increase worldwide, because of the large use of these drugs in patients with breast or gastrointestinal cancers in the near future. [11] The symptoms of toxicity vary from asymptomatic electrocardiographic changes to life-threatening events. Apart from the past history of ischemic cardiac disorders, no other risk factors have been identified.
Conclusion
Fluoropyrimidine rechallenge may be considered after a first cardiac episode, but it should be proposed with great caution. Education regarding the side effects of cardiovascular impairment should be informed to the patients and caregivers about the side effects in order to assess the risks and benefits of the medication.
REFERENCES
1.
Cardinale D, Colombo A, Colombo N. Acute coronary syndrome induced by oral capecitabine. Can J Cardiol 2006 Mar 1;22(3):251–3. [CrossRef]
[Pubmed]

2.
Hoff PM. Practical considerations in the use of oral fluoropyrimidines. Semin Oncol 2003 Jun;30(3 Suppl 6):88–92. [CrossRef]
[Pubmed]

3.
Labianca R, Beretta G, Clerici M, Fraschini P, Luporini G. Cardiac toxicity of 5-fluorouracil: A study on 1083 patients. Tumori 1982 Dec 31;68(6):505–10.
[Pubmed]

4.
Bertolini A, Flumanò M, Fusco O, et al. Acute cardiotoxicity during capecitabine treatment: A case report. Tumori 2001 May–Jun;87(3):200–6.
[Pubmed]

5.
Jensen SA, Sørensen JB. Risk factors and prevention of cardiotoxicity induced by 5-fluorouracil or capecitabine. Cancer Chemother Pharmacol 2006 Oct;58(4):487–93. [CrossRef]
[Pubmed]

6.
Kosmas C, Kallistratos MS, Kopterides P, Cardiotoxicity of fluoropyrimidines in different schedules of administration: A prospective study. J Cancer Res Clin Oncol 2008 Jan;134(1):75–82. [CrossRef]
[Pubmed]

7.
Kosmas C, Kallistratos MS, Kopterides P, Cardiotoxicity of fluoropyrimidines in different schedules of administration: A prospective study. J Cancer Res Clin Oncol 2008 Jan;134(1):75–82. [CrossRef]
[Pubmed]

8.
Eskilsson J, Albertsson M. Failure of preventing 5-fluorouracil cardiotoxicity by prophylactic treatment with verapamil. Acta Oncol 1990;29(8):1001–3. [CrossRef]
[Pubmed]

9.
Polk A, Vaage-Nilsen M, Vistisen K, Nielsen DL. Cardiotoxicity in cancer patients treated with 5-fluorouracil or capecitabine: A systematic review of incidence, manifestations and predisposing factors. Cancer Treat Rev 2013 Dec;39(8):974–84. [CrossRef]
[Pubmed]

10.
Koca D, Salman T, Unek IT, et al. Clinical and electrocardiography changes in patients treated with capecitabine. Chemotherapy 2011;57(5):381–7. [CrossRef]
[Pubmed]

11.
Meyer CC, Calis KA, Burke LB, Walawander CA, Grasela TH. Symptomatic cardiotoxicity associated with 5-fluorouracil. Pharmacotherapy 1997 Jul–Aug;17(4):729–36. [CrossRef]
[Pubmed]

SUPPORTING INFORMATION
Author Contributions
Nitish Kumar Sharma - Substantial contributions to conception and design, Acquisition of data, Analysis of data, Interpretation of data, Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published
Parth Shah - Substantial contributions to conception and design, Acquisition of data, Analysis of data, Interpretation of data, Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published
Ajay Sharma - Substantial contributions to conception and design, Acquisition of data, Analysis of data, Interpretation of data, Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published
Sarju Ganatra - Substantial contributions to conception and design, Acquisition of data, Analysis of data, Interpretation of data, Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published
Guarantor of SubmissionThe corresponding author is the guarantor of submission.
Source of SupportNone
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Conflict of InterestAuthors declare no conflict of interest.
Copyright© 2018 Nitish Kumar Sharma et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.