| Table of Contents |  |
|
Review Article
|
| Cardiovascular adverse effects of metoclopramide: Review of literature |
| Martha M Rumore1 |
|
1Assistant Director, Pharmacy Clinical & Educational Services, Cohen Children's Medical Center, North Shore-LIJ, New Hyde Park, N.Y. and Adjunct Professor, Pharmacy & Health Outcomes, Touro College of Pharmacy, New York, N.Y.
|
|
doi:10.5348/ijcri-2012-05-116-RA-1
|
|
Address correspondence to: Dr. Martha M Rumore Cohen Children's Medical Center 420 Lakeville Road New Hyde Park NY - 11040 Phone: Attn: Pharmacy Dept., Ph: (516) 470-8643 Email: mrumore@nshs.edu |
|
[HTML Abstract]
[PDF Full Text]
|
| How to cite this article: |
| Rumore MM. Cardiovascular adverse effects of metoclopramide: Review of literature. International Journal of Case Reports and Images 2012;3(5):1–10. |
|
Abstract
|
|
Introduction:
Articles pertaining to reports and clinical or pharmacological research for cardiovascular adverse effects of metoclopramide were identified via a search of MEDLINE (1966-February 2012), SCOPUS, and Google Scholar.
Objective: To review the reports in literature of patients receiving metoclopramide who sufferred from cardiac arrest, bradycardia, total heart block, acute hypotension, supraventricular tachycardia, circulatory collapse, QT prolongation, Torsade de Pointes, ST depression, and congestive heart failure. Discussion: In most cases the reactions occurred immediately after administration of metoclopramide, were associated with normal doses administered via intravenous or central lines, and resolved. Rechallenge occurred in several cases. The likelihood of these events occurring and the mechanism by which metoclopramide affects the cardiovascular system is unclear, however, it has been shown to have a direct effect on the heart, block presynaptic autoreceptors and enhance catecholamine release, enhance cholinergic neurotransmission and cause 5-HT3 receptor blockade and 5-HT4 receptor antagonism. Conclusion: Due to cardiovascular risks associated with the use of IV metoclopramide, recommendations are to monitor patients and report these events. | |
|
Key Words:
Metoclopramide, Cardiovascular, Adverse drug reactions, Case reports, Cardiac
| |
|
Introduction
| ||||||
|
Metoclopramide (1,4-amino-5-chloro-2 methoxy-N- (2-diethyl-aminoethyl) benzamide), a dopaminergic antagonist structurally related to procainamide, is an effective agent in treating and preventing vomiting and is useful in esophageal reflux disease, gastroparesis, dyspepsia and other gastrointestinal disorders. It promotes gastric emptying prior to anesthesia and reduces post-operative vomiting, possibly by blocking the chemoreceptor trigger zone for vomiting. [1] Although metoclopramide has been available for decades, its side effect profile continues to evolve. In 2009, the Food and Drug Administration (FDA) required a Black Box warning regarding the increased risk of tardive dyskinesia when metoclopramide is used at high doses or over long periods of time. Today, over 1,000 lawsuits claiming tardive dyskinesia from metoclopramide are pending in New Jersey courts alone. [2] Reports of cardiovascular adverse effects from metoclopramide first appeared in the literature in 1974, when Shaklai et al. noted cardiac arrhythmias, specifically multifocal preventricular contractions (PVCs) which resolved within one hour and reoccurred upon rechallenge. [3] Since then, a number of case reports have appeared in literature. | ||||||
|
Objective
| ||||||
|
The aim of this study is to review all available reports and patient series of metoclopramide induced cardiovascular (CV) adverse effects and provide recommendations for clinicians. | ||||||
|
Discussion
| ||||||
|
Metoclopramide is a benzamide derivative having various physiologic effects with side effects occurring in 10-20% of patients. [4] The most common side effects include drowsiness, GI disturbances, extrapyramidal reactions and increased lactation. [5] Metoclopramide has long been considered to lack significant CV effects. However, conflicting literature for CV effects of metoclopramide has appeared and will be discussed. In large doses in patients with heart disease, metoclopramide had no marked influence on hemodynamic parameters. [6] In animal studies, metoclopramide had a negligible effect on blood pressure (BP) responses to acetylcholine, adrenaline, histamine and noradrenaline. [7] The drug has been found to block the hypotensive action of dopamine. Cardiac conduction is unaffected by metoclopramide, but in animal studies large doses prevented experimentally-induced cardiac arrhythmias and produced a slight blood pressure decrease. [1] Careful consideration should be given to examine the risks and benefits of using metoclopramide for preoperative prophylaxis of nausea and vomiting. Study Method: To identify pertinent literature, including but not limited to case reports describing CV adverse reactions from use of metoclopramide, we conducted a search using MEDLINE (1966- to February 2012), SCOPUS and Google Scholar engine using the search terms: adverse effects, metoclopramide, cardiac, cardiovascular, cardiac arrest, sinus arrest, bradycardia, tachycardia, arrhythmia, QT prolongation, Torsade de Pointes, hypotension and a combination of text and MeSH terms. Bibliographies of identified articles were subsequently reviewed for additional citations. Review of Cases in the Literature: Cases of sinus or cardiac arrest, [8] [9] [10] [11] [12] [13] [14] [15] bradycardia followed by total heart block, [14] [16] [17] [18] acute hypotension, [19] [20] [21] [22] [23] [24] and circulatory collapse [24] have been reported with metoclopramide. The duration of the cardiac arrest reported in the cases is mostly between 15–30 sec, but on one occasion it lasted two min. [11] Several cases describe extreme bradycardia. [9] [10] [12] [14] [16] Others have reported cardiac arrhythmias such as QT prolongation, [25] [26] [27] Torsade de Pointes, [13] [26] [28] [29] supraventricular tachycardia (SVT) [28] [30] [31] [32] and ST depression. [28] [30] Cases of congestive heart failure have occurred following chronic metoclopramide at doses of 40 mg/day [31]. In patients with heart failure, metoclopramide at doses of 10 mg three times a day blunted the natriuretic response to saline load. [34] The authors concluded that metoclopramide should be used with caution in heart failure patients and those with volume overload. Single IV doses of 10 mg decreased diastolic BP in women with pregnancy-induced hypertension and increased plasma aldosterone in every subject. [35] However, the increase in plasma aldosterone was greater in women with pregnancy-induced hypertension than in normal pregnant women (p < 0.05). Congestive heart failure is most likely due to a different mechanism of action as dopaminergic inhibition is thought to result in increases in plasma aldosterone, thereby producing sodium retention. [30] The summary of key cases retrieved is presented in table 1. | ||||||
| ||||||
|
A number of cardiovascular adverse effects are listed in the metoclopramide prescribing information, including AV block, bradycardia, heart failure, hypertension/hypotension, SVT but not circulatory collapse, cardiac arrest, Torsade de Pointes, QT prolongation, and ST depression. [36] Rechallenge with metoclopramide occurred in a number of cases. Lau et al. reported two separate episodes of circulatory collapse following IV metoclopramide. [24] Bentsen et al. reported five repeated episodes of cardiac arrest following five separate metoclopramide injections over 48 hours. [11] Unusual drug reactions may arise as a result of multiple factors. Suggested underlying predisposing or contributory factors for development of cardiac adverse effects with metoclopamide include previous cardiovascular disease, atrial fibrillation, [8] autonomous dysfunction, [15] hyperbilirubinemia, [11] halothane anesthesia, [19] [21] and pericardial drainage tube. [16] However, in other cases there has been no clear association with any risk factors. For example, cardiac arrest following metoclopramide has been reported in patients without known cardiac disease. [13] Risk Factors:Neither age nor dose appears to be contributory factors for cardiovascular adverse reactions from metoclopramide. While the patients in most of the cases were elderly, cardiac arrest, for example, following metoclopramide has occurred in middle-aged individuals as well. [11] However, no case reports in pediatric patients were found. Rose et al. observed that rapidly administered IV metoclopramide at 0.25 mg/kg had no effect on either heart rate or BP in 45 children between the ages of 2–16 years old prior to elective surgery. [37] However, in adult patients, Blanco et al. observed that IV metoclopramide decreased BP in both normotensive and hypertensive adults with greater decreases in the later. [38] Other studies have also demonstrated vascular hyperreactivity during cold presser test (CPT) with metoclopramide at doses of 7.5 mcg/kg/min during a 30 minute period in both normotensive and hypertensive patients. [39] Metoclopramide significantly decreased BP but did not alter heart rate (HR). The authors attributed this effect to an a-adrenergic blocking effect but not to an antidopaminergic effect. In labetalol pre-treated hypertensive patients, HR increased about 11.5 beats per minute during CPT. BP increased from a mean of 157/97 mmHg to 193/123 mmHg (+36.8/+25.7 mmHg). [40] The BP response was blocked by bromocriptine, a known D2-dopaminergic agonist. Moreover, the cardiovascular adverse effects do not appear to be a dose-related effect they occurred after single doses ranging from 2.5–10 mg IV. [13] [15] [16] The route of administration may be a causative factor. In all cases, metoclopramide was given IV either peripherally or via a central venous line. The rate of injection may also be causative. In a number of cases, metoclopramide was administered over seconds, not over 1–2 minutes. [20] In one study in a series of 16 patients, doses of 10 mg IV produced average decreases in systolic and diastolic BP of 22% and 20%, respectively, occurring on average 44 seconds after administration over a 10 second period. [21] The prescribing information specifically cautions that IV administration should be slow over 1–2 minutes. However, the reason stated in the package insert for slow injection is avoidance of "a transient but intense feeling of anxiety and restlessness, followed by drowsiness", and not to avoid rapid decreases in BP or cardiovascular adverse effects. [36] Potential Mechanisms Involved: Metoclopramide has potent central anti-dopaminergic and peripheral cholinergic effects. The mechanism(s) by which metoclopramide causes cardiovascular adverse effects such as cardiac arrest is not known but may be multifactorial involving: 1) a direct effect on the heart; 2) blockade of presynaptic autoreceptors and enhanced catecholamine release; [14] [42] 3) other actions caused by enhancement of cholinergic neurotransmission; [5] or 4) 5-hydroxytryptamine (5-HT3) receptor blockade or 5-HT4 receptor agonism. [43] Support for a mechanism involving a direct effect of metoclopramide on the heart may be found in the structural similarity of metoclopramide with procainamide. Metoclopramide differs from procainamide by only a 2,5 aryl substitution. [5] Procainamide prolongs AV conduction and may produce tachycardia. Moreover, procainamide can also cause peripheral vasodilation. Procainamide and cholinergic stimulation, in general, have long been known to cause sinus arrest. [14] The effects of metoclopramide may be due to either a direct cardiac effect (i.e. myocardial depression) or vasodilation. However, the BP lowering effects of metoclopramide are unlikely to be due to a dopaminergic mechanism but may be caused by arteriolar vasodilation. Doses of 40 mg daily metoclopramide reduced HR and antagonized BP increases induced by treadmill exercise in normotensive subjects. [44] The first potential mechanism, a direct effect is likely to appear immediately after injection and may possibly be linked to sodium channel blocking antiarrhythmic effects. In four cases of cardiac arrest, the heart stopped within 30 seconds. [9] [10] [11] [16] However, in one case sinus arrest and bradycardia occurred 10–15 minutes after IV administration, which suggests a different mechanism. [45] With regard to the possible blockade of presynaptic autoreceptors by metoclopramide, it is known that metoclopramide enhances catecholamine release in patients with pheochromocytoma and "essential" hypertension. [46] [47] This results in a severe pressor reaction with increased BP and decreased HR and is believed to be, at least in part, mediated by vasopressin release. [41] Metoclopramide is known to have a striking influence on vasopressin secretion. [48] This effect is believed to be mediated by cholinergic stimulation. Vasopressin produces constriction of coronary arteries (thereby reducing oxygen delivery) and increases myocardial oxygen demand by increasing afterload on the heart. It also increases peripheral vascular resistance, which in turn, increases BP. In the past, the drug was actually used to treat orthostatic hypotension and vascular headaches because of its vasoconstrictive effects. [48] Metoclopramide 20 mg IV induces increased vasopressin levels in healthy patients and type 2 diabetics. [49] In one study in hypertensive patients, intraveneously administered metoclopramide was shown to release catecholamines. [41] One case of cardiac arrest occurred following discontinuation of dopamine. The long-standing influence of dopamine on the number and function of cardiac beta 1 receptors or the dopamine effect of release of norepinephrine in cardiac sympathetic nerve terminals and induced release of circulating catecholamines were postulated as possible contributory factors. Alternatively, metoclopramide releases acetylcholine from cholinergic nerve terminals inducing peripheral vasodilation. [43] Studies have shown that acetylcholine levels are increased after metoclopramide administration.[50] [51] [52] Additionally, in vitro metoclopramide inhibited acetylcholine to prevent its degradation. [53] Metoclopramide increases release of acetylcholine in the CNS possibly causing cholinergic-induced bradyarrhythmias. It is possible that the effect on the heart may be the result of changes in cholinergic tone mimicking vagal stimulation. [29] In animal studies, metoclopramide blocks cardiac dopamine receptors in rats and high doses in cats produces transient hypotensive effects. [54] [55] In other animal studies, doses of 10 mg/kg produced bradycardia. [45] [54] In dogs, doses of 10 mg/kg decreased systolic and diastolic blood pressure, left ventricular systolic pressure, and total peripheral resistance. [57] In humans, quinidine-like antiarrhythmic effects have been observed. [55] [56] Bozzi et al. observed that metoclopramide as a 20 mg bolus followed by 20 mg IV at a rate of 1 mg/min had no significant effect on sinus node conduction but did increase atrial and AV nodal refractory periods. [58] A potential fourth mechanism for CV effects of metoclopramide is 5-HT3 receptor blockade or 5-HT4 receptor agonism. In addition to its dopamine D2 receptor antagonist effects, metoclopramide is a mixed 5-HT3 receptor antagonist and 5-HT4 receptor agonist. Metoclopramide's peripheral action to increase lower esophageal tone and gastric emptying occurs via 5HT4 receptor stimulation, whereas the antiemetic effects may be attributed to 5HT3 receptor blockade. [59] [60] 5-HT3 receptor blockage could influence serotonin (Bezold-Jarish reflex) causing bradycardia and hypotension. [61] Metoclopramide has also been shown to antagonize serotonin-induced bradycardia. [62] Lau attributed circulatory collapse from metoclopramide to excessive serotonin autoinhibition. [24] 5-HT4 receptors are also located in the heart and the vasculature where they exert a positive chronotropic effect and tachycardia by an action on the atrium. 5-HT4 receptor agonists are well known to exert their side effects mainly on the lower urinary tract and the CV system. Metoclopramide is structurally related to cisapride, both being gastric prokinetic substituted benzamides. Cisapride was known to trigger tachycardia and SVT through stimulation of 5-HT4 atrial receptors. The cardiotoxic potential of cisapride was mainly due to QT prolongation and development of Torsades de Pointes. This effect was deemed especially problematic in patients concomitantly treated with drugs known to inhibit the CYP3A4 isoenzyme. [63] [64] As recently as five years ago, a new metabolic pathway for metoclopramide involving the CYP2D6 isoenzyme was identified. [65] In 100 healthy subjects, 10 mg of metoclopramide prolonged the QT interval from 13.2±1 to 19±1 m sec. [27] Elimination of metoclopramide takes place through hepatic metabolism involving CYP2D6. Metoclopramide elimination is likely to be slowed in poor metabolizers of CYP2D6 or those taking inhibitors of this isoform such as omeprazole. [64] It has been suggested that a toxic metabolite of metoclopramide may be linked to the tardive dyskinesia from this agent. [66] Whether or not the CV adverse effects from metoclopramide can also be linked to the CYP isoenzymes is unknown. However, since the CV adverse effects in most cases occurred immediately, a metabolic effect is deemed unlikely to have been causative. Future Research: It is unlikely that further research will be undertaken as metoclopramide is available generically and there are over 10 manufacturers listed in the Orange Book. In summary, metoclopramide may occasionally cause bradyarrhythmias progressing to cardiac arrest, paroxysmal SVT, hypotension, circulatory collapse, QT prolongation, Torsade de Pointes, heart block, and hypotension in patients without evidence of underlying functional or structural cardiac abnormalities | ||||||
|
Conclusion
| ||||||
|
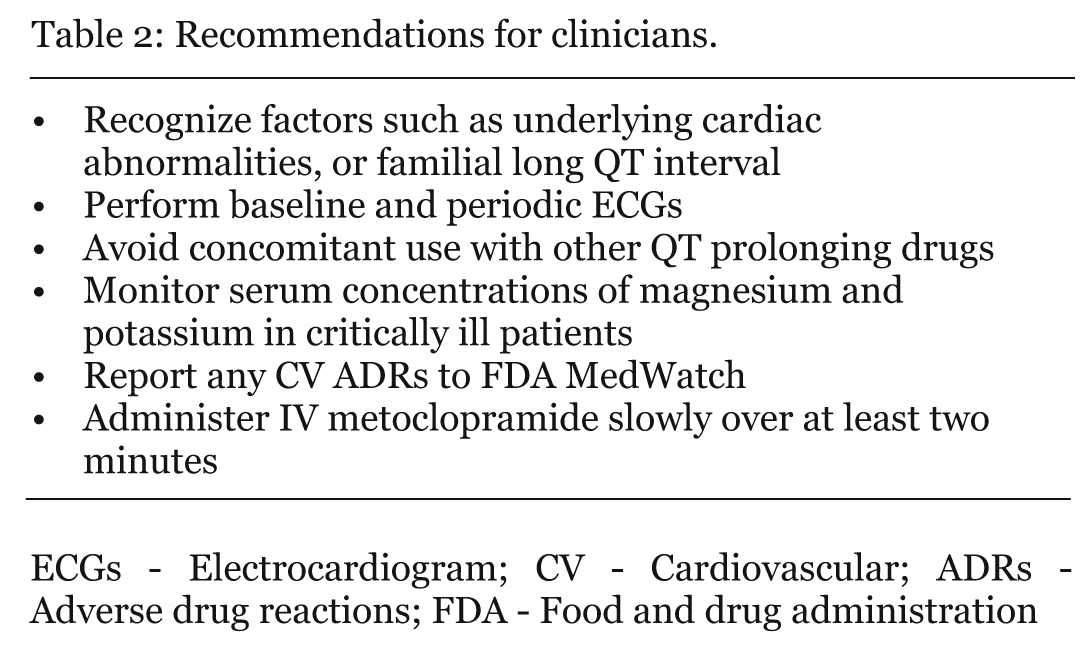
Cardiovascular CV adverse effects of metoclopramide are rare but some can be fatal. It would appear to be prudent to monitor patients receiving metoclopramide IV immediately after injection for CV adverse effects. Due to the possibility of CV risks associated with metoclopramide, it is important for clinicians to take at least some of the steps outlined in table 2. We believe it is also appropriate to warn against rapid IV injection, especially via the central venous route. Postmarketing surveillance has helped to identify rare side effects of drugs after they are on the market, but the occurrence of CV adverse effects such as bradycardia and cardiac arrest from metoclopramide which may be ascribed to an underlying disease, are probably underreported. Further, in view of the number of cases reported, we recommend additional CV adverse effects be included in the "Adverse Reactions" section of the Prescribing Information for metoclopramide. Rarely, poorly understood side effects occur with many highly effective drugs. This review should prompt a critical re-evaluation of the risk/benefit of metoclopramide and consideration of therapeutic alternatives, in concert with a search for underlying predisposing factors or mechanisms of action involved. | ||||||
| ||||||
|
References
| ||||||
| ||||||
|
[HTML Abstract]
[PDF Full Text]
|
|
Author Contributions:
Martha M Rumore - Conception and design, Acquisition of data, Analysis and interpretation of data, Drafting the article, Critical Review of the Article, Final approval of the version to be published |
|
Guarantor of submission:
The corresponding author is the guarantor of submission. |
|
Source of support:
None |
|
Conflict of interest:
Authors declare no conflict of interest. |
|
Copyright:
© Martha M Rumore 2012; This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any means provided the original authors and original publisher are properly credited. (Please see Copyright Policy for more information.) |
|
|