|
Case Series
Pneumomediastinum in COVID-19 critically ill patients: A case series of unusual complication from a Tunisian intensive care unit
1 Medical Intensive Care Unit, Ibn El Jazzar University Hospital, Kairouan, Tunisia
2 Pulmonology Department, El Jazzar University Hospital, Kairouan, Tunisia
Address correspondence to:
Jihene Ayachi
Road Iskander Makdouni n°17, Kairouan 3100,
Tunisia
Message to Corresponding Author
Article ID: 101326Z01DB2022
Access full text article on other devices

Access PDF of article on other devices

How to cite this article
Braiek DB, Mighri I, Zorgati H, Jazia RB, Kacem A, Ayachi J. Pneumomediastinum in COVID-19 critically ill patients: A case series of unusual complication from a Tunisian intensive care unit. Int J Case Rep Images 2022;13(2):50–57.ABSTRACT
Introduction: Coronavirus disease 2019 (COVID-19) is a new rapidly emerging and ever-evolving disease that clinicians continue to detect new manifestations and complications daily since December 2019. Pneumomediastinum, a potentially life-threatening condition, is an uncommon complication of acute respiratory distress syndrome from viral infections. By presenting this case series, we highlight that pneumomediastinum (PM) can complicate the course of a severe COVID-19 infection.
Case Series: We identified four critically ill patients, two men and two women, aged between 50 and 70 years old. None of them had any underlying lung disease. On admission all cases were in acute respiratory distress syndrome. Three patients were under positive pressure ventilation both invasive (n = 2/4) and non-invasive (n = 1/4) at the time of the event; however, one patient had a spontaneous PM without any exposure to mechanical ventilation. Chest computed tomography scan (chest CT scan) was performed for all patients showing a pulmonary involvement estimated moderate (n = 3/4) to severe (n = 1/4), PM (n = 4/4) and subcutaneous emphysema (n = 2/4). For ventilated patients, PM was diagnosed 3 to 7 days after initiation of mechanical ventilation. The highest positive end-expiratory pressure was 10 cmH2O for patients receiving invasive mechanical ventilation, while 5 cmH2O for patient who had developed PM on non-invasive ventilation. The PM was managed by conservative therapy in all of the cases with reducing airway pressure.
Conclusion: Our findings suggest that PM is secondary to inflammatory response due to COVID-19 and mostly triggered by the use of positive pressure ventilation and it is associated with poor outcome in critically ill COVID-19 patients.
Keywords: COVID-19, Intensive care unit, Pneumomediastinum, Positive pressure respiration
Introduction
Since December 2019, coronavirus disease 2019 (COVID-19) has rapidly spread worldwide and it has become a global pandemic in March 2020 [1]. Given current knowledge of this ever-evolving disease, it is important to highlight its uncommon complications so that adverse events could be better predicted and treated appropriately. Pneumomediastinum (PM) has been recently reported as an unusual complication in cases with COVID-19 pneumonia that can potentially be an aggravating factor [2],[3].
Case Report
We identified four patients with PM complicating the course of a severe coronavirus disease 2019 (COVID-19) infection. They were two men and two women, aged between 50 and 70 years old. A case-by-case review of the four clinical presentations is detailed below, with summarizing table of all cases (Table 1).
CASE 1: (Spontaneous Pneumomediastinum)
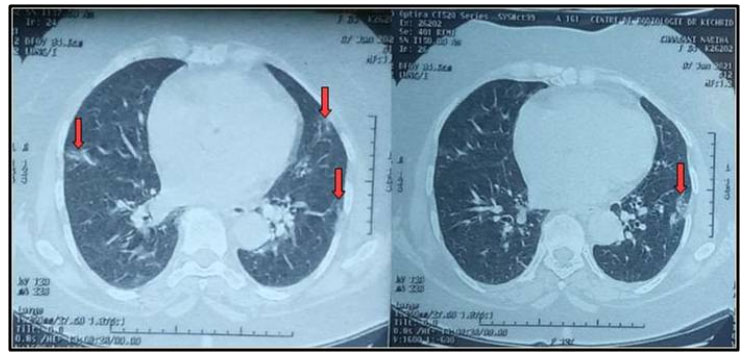
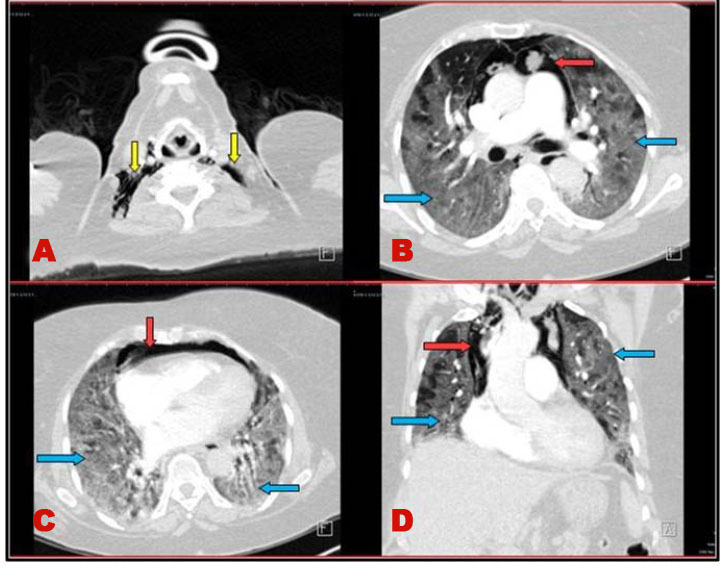
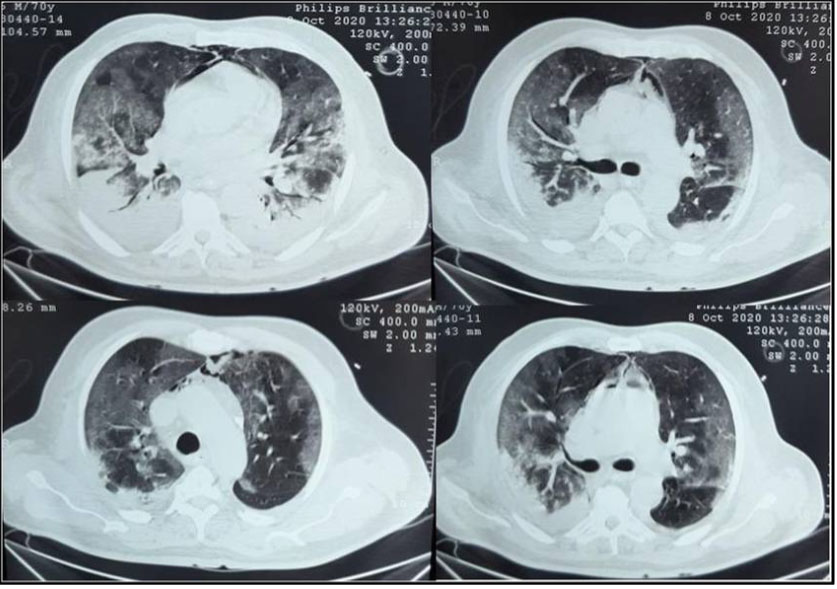
A 65-year-old female patient with medical past history of obesity (body mass index (BMI) at 35 kg/m2), hypothyroidism treated by levothyroxine replacement therapy, was admitted in pulmonology department for confirmed COVID-19 pneumonia with two weeks history of fever, chills, asthenia, dry cough, and progressive shortness of breath, with initial non-contrast chest computed tomography scan (chest CT scan) showing bilateral peripheral ground-glass opacities and crazy paving with estimated percentage of lung involvement at 10% (Figure 1). She was managed initially with oxygen-therapy with nasal prongs associated to antibiotics, dexamethasone 6 mg/12 h, anticoagulation, hydration, and vitamins. On day four of admission, the patient developed a rapid onset of retrosternal chest pain associated to a moderate acute respiratory failure with severe hypoxemia: oxygen saturation (SpO2) of 88% under 15 L/min of oxygen with a non-rebreather mask. In front of the worsening of her state, she was transferred to our intensive care unit (ICU). Upon admission, her respiratory rate (RR) was of 35 breaths/min with struggle signs and hypoxemia and soft-tissue crepitus bilaterally. She was conscious with stable hemodynamic state. Laboratory tests revealed an elevated C-reactive protein (CRP) concentration of 53 mg/L (reference:<5 mg/L). Complete blood count showed a leukocytosis of 11.8×109/L (reference: 4–10×109/L), with lymphopenia (0.4×109/L). She had hepatic cytolysis with alanine aminotransferase (ALAT) and aspartate aminotransferase (ASAT) at 100 IU/L (reference:<45 IU/L) and 68 IU/L (reference: <50 IU/L) respectively. Serum level of D-dimer was at 457 µg/L (reference:< 500 µg/L). Arterial blood gas (ABG) showed a pH:7.41, PaCO2:43 mmHg, PaO2:74 mmHg, HCO3:27 mmol/L and PaO2/FiO2 ratio at 205. A contrast-enhanced chest CT scan was performed showing diffuse lower neck subcutaneous emphysema associated with diffuse PM and diffuse bilateral ground-glass opacities with posterior and peripheral predominance associated to alveolar consolidation with estimated percentage of lung involvement at 90% consistent with critical COVID-19 pneumonia (Figure 2A, Figure 2B, Figure 2C, Figure 2D). Urgent mediastinal decompression with chest drainage tube wasn’t immediately indicated in front of the maintained stable hemodynamic state, so we decided the conservative therapy with reduced airway pressure with non-invasive ventilation (NIV) [pressure support (PS) of 12–14 cmH2O, positive end expiratory pressure (PEEP) of 4 cmH2O, and fractional inspired oxygen (FiO2) of 100%]; awake prone positioning and close monitoring. Initially we obtained a slight improvement of his respiratory state. At day 7 of ICU stay she was intubated in front of NIV failure. The patient was ventilated with protective ventilation with volume assist-control ventilation mode (VAC mode) [tidal volume (Vt) at 6 mL/kg predicted body weight (PBW), PEEP at 4 cmH2O: low level in front of the PM, respiratory rate at 30 breaths/min and 100% of FiO2. She was immediately put under continuous infusion of sedation and neuromuscular blockers. Despite protective ventilation with lower pressure, there was a maintained severe hypoxemia, and the patient passed away due to refractory hypoxemia few hours later.
CASE 2: (NIV-induced pneumomediastinum)
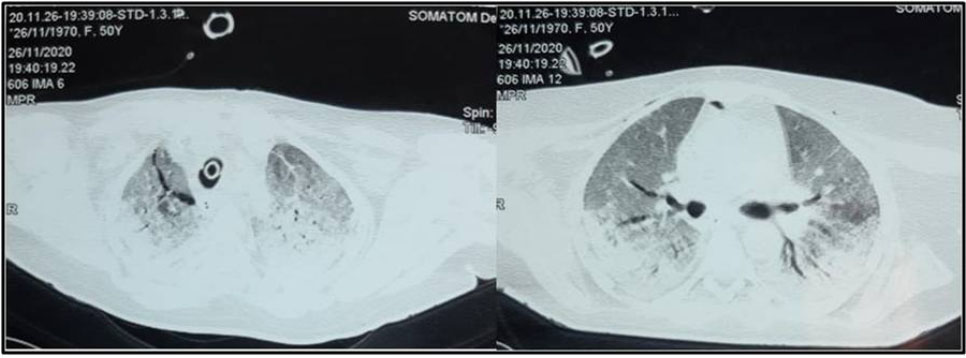
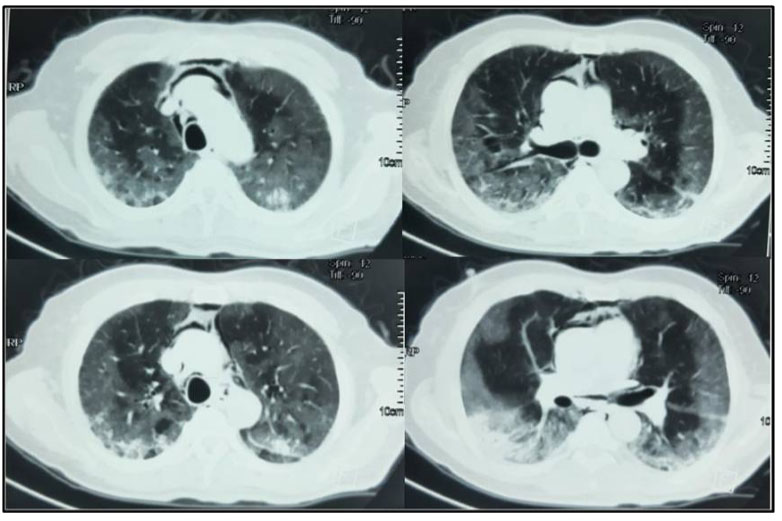
A 61-year-old male patient with past medical history of diabetes was admitted in the pulmonology department for COVID-19 pneumonia. On examination the patient was conscious, febrile at 38.5°C, respiratory rate of 25 breaths/min, hypoxemic with SpO2 on room air of 80% with bilateral crackles on pulmonary auscultation and with stable hemodynamic state. Blood test results showed CRP of 80 mg/L and leukocytes of 8300 cells/mm3. Chest X-ray showed bilateral alveolar and interstitial opacities. A chest CT scan was performed showing bilateral ground-glass opacities with estimated lesions of 30%. He was put under 10 L/min of oxygen with non-rebreather mask with SpO2 of 96%; antibiotics; dexamethasone: 6 mg/day, preventive anticoagulation, famotidine, and vitamins. The patient was transferred three days later to our ICU in front of worsening of his respiratory state with SpO2 of 80% under 15 L/min of oxygen, respiratory rate of 35 breaths/min with struggle signs. He was put under NIV with FiO2: 70–100%, PS: 16–20 cmH2O and PEEP: 6 cmH2O and prone position. A control chest CT scan was performed four days later (after NIV use and slight improvement of the respiratory state) showing: worsening of the bilateral ground-glass opacities with percentage of lesions at 75% associated to a moderate PM (Figure 3). Conservative therapy was adopted with decreasing pressures at NIV (PS: 12–14 cmH2O, PEEP: 4 cmH2O) alternately with high flow nasal cannula oxygenation (HFNC) (flow 50 L/min and FiO2 100%). Unfortunately, five days later, the patient was intubated ventilated under protective ventilation in front of NIV failure with VAC mode Vt: 6 mL/kg of PBW, RR: 26 breaths/min, PEEP: 8 cmH2O and FiO2: 100%. The patient passed away 15 days later in a severe acute respiratory distress syndrome (ARDS) with refractory hypoxemia and shock.
CASE 3: [Invasive mechanical ventilation (IMV) induced PM]
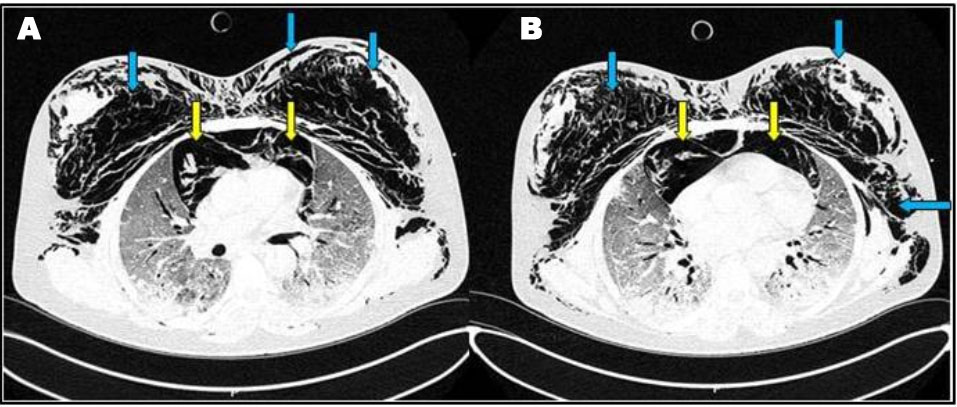
A 50-year-old female patient with past history of obesity with BMI at 30 kg/m2 was admitted in our ICU for acute respiratory failure due to COVID-19 pneumonia. There was a history of dyspnea asthenia and cough one week before ICU admission. The patient was initially managed in the pulmonology department with non-rebreather oxygen mask. A non-contrast chest CT scan was performed showing diffuse bilateral ground-glass opacities with posterior and peripheral predominance associated to alveolar consolidation with estimated percentage of lesion at 75% consistent with severe COVID-19 pneumonia (Figure 4A). In front of worsening of his respiratory state, she was transferred to our ICU with moderate acute respiratory failure. Upon admission, her respiratory rate was of 40 breaths/min with struggle signs, SpO2 of 88% under 15 L/min of oxygen with non-rebreather mask, she was conscious with stable hemodynamic state. Blood test results showed a CRP of 81 mg/L, leukocytes of 18.400/mm3, D-dimer of 290 µg/L and the ABG showed respiratory alkalosis with pH: 7.47, PaO2: 51 mmHg, PaCO2: 32 mmHg, HCO3−: 23 mmol/L, and SaO2: 88%. The patient was put initially under NIV (PS of 20 cmH2O, PEEP of 6 cmH2O, and FiO2 100%); antibiotics, dexamethasone 6 mg/12 h, anticoagulation and prone positioning. At day 6 of ICU stay she was intubated in front of NIV failure using direct laryngoscopy with no difficult airway management, no bougie or stylet were used and 7.5 cm tracheal tube was inserted at level 23 of dental arch. The patient was ventilated with protective ventilation with VAC mode (Vt: 6 mL/kg of PBW, PEEP: 10 cmH2O, RR: 26 breaths/min, and FiO2 100%). Under protective ventilation, peak airway pressure (PIC) was of 28 cmH2O, plateau pressure (P.Plat) of 24 cmH2O, and driving pressure (DP) of 14 cmH2O. She was immediately put under continuous infusion of sedation and neuromuscular blockers. Four hours after intubation a subcutaneous emphysema appeared in the neck, chest wall, and in the abdomen. On examination there was a symmetric crackle on thoracic auscultation without silence, respiratory mechanics showed increasing in PIC of 35 cmH2O and P.Plat of 30 cmH2O without worsening of hemodynamic state. A non-contrast chest CT scan was performed showing: huge PM reaching the right peritoneal space associated with an important subcutaneous emphysema in the neck and thoraco-abdominal region with suspected tracheal perforation with worsening of the ground-glass opacities and inferior alveolar consolidation, no pneumothorax was associated (Figure 4B). In front of the deep hypoxemia (PaO2/FiO2 of 80 and SpO2 at 86% with deep decrease of SpO2 till 50% while ventilator disconnection attempts), a flexible bronchoscopy to identify the tracheal breach wasn't possible. The attitude was to introduce the tracheal tube till 27 level of dental arch to plug the tracheal breach with the balloon (according to the CT thoracic scan data we recommend us to introduce the tracheal tube by 4 cm approximately), decreasing the PEEP to 6 cmH2O with close monitoring of the hemodynamic and respiratory state. The evolution was marked by the regression of the subcutaneous emphysema five days later and the improving of the respiratory state (PaO2/FiO2 of 200, SpO2 at 95% and peak pressure of 28 cmH2O). A non-contrast chest CT control scan was performed showing a significant regression of the PM and the subcutaneous emphysema with persistent severe lung lesion with ground-glass opacities and consolidation images (Figure 5). The patient was maintained under protective ventilation with difficult weaning. The evolution was marked by a septic shock due to ventilator acquired pneumonia, and she passed away at day 18 of ICU stay in a refractory shock.
CASE 4: (IMV-induced pneumomediastinum)
A 70-year-old male patient with past medical history of diabetes type 2 was admitted in our ICU for acute respiratory failure due to COVID-19 pneumonia with diffuse bilateral ground-glass opacities on non-contrast chest CT scan. Upon admission, his respiratory rate was of 26 breaths/min, SpO2 at 88% under 15 L/min of oxygen with non-rebreather mask, he was conscious with stable hemodynamic state. Blood test results showed a CRP of 90 mg/L, leukocytes of 15,000/mm3. His ABG showed pH: 7.38, PaO2: 49 mmHg, PaCO2: 29 mmHg, HCO3−: 17.2 mmol/L and SaO2: 83%. The patient was maintained under 15 L/min of oxygen with SpO2 95% and RR 20–25 breaths/min, put under antibiotics; Dexamethasone 6 mg/12 h, anticoagulation and prone positioning. After 24 hours of ICU stay, the patient was intubated in front of severe acute respiratory failure. The trachea was intubated using direct laryngoscopy with no difficult airway management, no bougie or stylet were used, 7.5 cm tracheal tube was inserted, the patient was ventilated with protective ventilation with VAC mode (Vt: 6 mL/kg of PBW, PEP: 10 mmHg, RR: 26 breaths/min, and FiO2: 100% with PIC at 28, P.Plat 25, and DP at 15); and immediately put under continuous infusion of sedation and muscle blockers. The evolution was marked initially by the deep hypoxemia with PaO2/FiO2 at 100, instable hemodynamic state and elevated PIC at 45 cmH2O. Then in front of the slight improvement of the respiratory and hemodynamic state, a control non-contrast chest CT scan was performed at day 6 after invasive mechanical ventilation showing: mild to moderate PM associated to mild bilateral pleural effusion with stable aspect of the ground-glass opacities associated to inferior alveolar consolidation (Figure 6). In front of the maintained stable hemodynamic state, we decided the conservative therapy with reducing airway pressure (PEEP at 5 cmH2O) and close monitoring. The patient was maintained under protective ventilation, sessions of prone position with difficult weaning. The evolution was marked by a septic shock, and he passed away at 18 of ICU stay.
Discussion
Coronavirus disease (COVID-19) is an infection caused by a newly identified coronavirus, first detected in Wuhan, China, in December 2019. It has become a pandemic and is posing a serious worldwide public health problem [4]. The clinical symptoms of COVID-19 and its complications are evolving. Pneumomediastinum (PM) is a rare clinical finding, but during COVID-19 pandemic it became the source of significant concern for clinicians [3]. The reported incidence of PM in conjunction with pulmonary infections by pathogens like severe acute respiratory syndrome (SARS), Middle East respiratory syndrome (MERS) was 1.7–12%, either spontaneous or associated to ventilation [5].
Recently, some sporadic cases of PM complicating the course of COVID-19 with varying outcomes have been reported; however, its incidence is still unknown [4],[6],[7],[8],[9],[10],[11].
To the best of our knowledge, this is the first case series describing pneumomediastinum in COVID-19 critically ill patients from Tunisia.
Pneumomediastinum is more frequent in males [9]. Tobacco, drug abuse, and several structural lung diseases, including chronic obstructive lung disease, asthma, and interstitial lung diseases are some predisposing factors [12]. In our case series, the sex ratio was 1:1 and none of patients had a risk factor in developing one of this complication.
Pneumomediastinum can be spontaneous, if the cause is idiopathic, or secondary if it responds to a known etiology, whether traumatic or iatrogenic [9]. Spontaneous pneumomediastinum (SPM) could be attributed to the Macklin effect [13]. This pathophysiological process has been interpreted as the effect of the peribronchiolar inflammatory nodule formation, leading to interstitial pulmonary emphysema, tracking back along the bronchovascular sheath and reaching the mediastinum [5]. Furthermore, histological findings (alveolar damage with pulmonary edema and hyaline membrane formation) appear to support the hypothesis that severe pulmonary injury predisposes the patient to SPM (Case 1) [5]. All patients in our case series had extensive bilateral pneumonia. According to a published score for quantifying the extent of SARS-CoV-2 pneumonia, one patient was “severe” and the others were “moderate” [14]. Forceful coughing with sudden alveolar over-distension can increase intra-alveolar pressure and rupture the alveoli in patients with COVID-19. Case 1 with SPM had intense repetitive episodes of dry cough during two weeks before the ICU admission. These coughing spells can be exerted significant strain on the already injured alveoli initiating the air leakage.
In the context of the severe respiratory disease associated with COVID-19, it is highly likely that the pathogenesis of the secondary PM is due to alveolar rupture secondary to barotrauma associated with mechanical ventilation due to higher pressure or volume lung ventilation strategies and loss of lung compliance due to the significant alveolar damage [8],[12].
Non-invasive ventilation (NIV) is used frequently for acute respiratory failure. It is therefore suggested that COVID-19 patients with extensive lung damage might have increased respiratory drive with persistent strong spontaneous inspiratory efforts causing self-inflicted lung injury as postulated by Gattinoni [15],[16]. The use of end expiratory pressure in NIV induce an increase of the gradient of pressure between alveoli and interstitium, which induce over-distension of already damaged alveoli causing their rupture with extension of air into subcutaneous tissue and mediastinum (Case 3) [10].
For invasively ventilated patients, Wali et al. [12] speculated that tracheal edema associated with viral infection may leave patients potentially vulnerable to iatrogenic tracheal injury specially during expeditious intubation due to severe hypoxemia in emergent settings, endotracheal suctioning, manipulation of tracheal tube during prone and supine positioning session (Case 4).
Typically, PM is considered as a benign entity; however, it can be life-threatening and potentially lead to further problems including cardiac tamponade, tension pneumothorax, and significant subcutaneous emphysema [17]. Therefore, PM should be closely monitored since it can lead to cardiorespiratory compromise due to diminished cardiac output by direct cardiac compression or reduced venous return [17].
The management of PM should be carried out alongside treatment of ongoing COVID-19 infection. Conservative approach is mostly adopted if possible based on rest, oxygen therapy and analgesia [3],[17]. When needed other options should be carried out including needle aspiration, chest drain insertion, fenestrated sub-cutaneous catheters, or vacuum-assisted closure dressings to the supra-clavicular space [12]. Due to the extension of air caudally, the most favorable location for decompression is superiorly, adjacent to the thoracic inlet [18].
If mechanical ventilation is indicated, patients should be ventilated with the least damaging settings possible to achieve adequate oxygenation in order to minimize the risk of barotrauma. In patients requiring escalating PEEP, efforts should be focused on identifying reversible causes and using strategies, as early prone position, to reduce PEEP [10]. For intubated patients, where major proximal tracheal injury is suspected, it is worth considering advancing the tracheal tube distal to the injury (Case 3). This could be better achieved by bronchoscopic control, but due the high risk of aerosolized viral exposure of the operator, bronchoscopy could be avoided.
Continued clinical and radiological assessment is mandatory to guide any urgent interventions. In severe and worsening cases of pneumomediastinum, associated with shift and respiratory compromise, it is recommended to insert subcutaneous drains and bilateral intra-pleural chest drains to prevent life-threatening complications. If life-threatening tamponade does occur, then immediate needle decompression is required [10].
While the PM is usually self-limiting in healthy individuals, its appearance in patients with severe acute respiratory syndrome (SARS) infection has been demonstrated as a potentially poor prognostic factor of disease severity, higher rates of intubation, and mortality. Whether the same applies to COVID-19 patients is a preoccupation. Recently, several case reports have described a poor prognosis in COVID-19 patients with PM patients [10].
Independently of mechanical ventilation, the occurrence of PM is related to increased risk of intubation and high mortality index. In their study, Manna et al. [19] reported that among 11 non-intubated COVID-19 patients with spontaneous PM and subcutaneous emphysema, 4 patients expired during the hospital stay. Studies into pulmonary barotrauma have suggested that PM is a negative prognostic indicator in intubated patients [12],[20]. Barotrauma in all its manifestations has been proven to increase mortality in patients with ARDS [12],[21].
Conclusion
Although relatively rare, our case series highlights the emerging association of COVID-19 infection with pneumomediastinum, either spontaneous or associated to ventilation, and the need to be alert to this complication in the evolution of the disease. Our findings suggest that development of PM in critically ill COVID-19 patients is associated with worse prognosis. Further studies are needed to better elucidate the risk factors associated with development of this complication and identify preventive and management measures for better outcomes in COVID-19 patients with PM.
REFERENCES
1.
Ramezani R, Jafari F, Fahami Y, Pakniyat A, Rad MG. A case report of pneumomediastinum and subcutaneous emphysema associated with pandemic COVID-19 in a 43-year-old man. Clin Imaging 2021;76:74–6. [CrossRef]
[Pubmed]

2.
Kolani S, Houari N, Haloua M, et al. Spontaneous pneumomediastinum occurring in the SARS-COV-2 infection. IDCases 2020;21:e00806. [CrossRef]
[Pubmed]

3.
Volpi S, Ali JM, Suleman A, Ahmed RN. Pneumomediastinum in COVID-19 patients: A case series of a rare complication. Eur J Cardiothorac Surg 2020;58(3):646–7. [CrossRef]
[Pubmed]

4.
Gorospe L, Ayala-Carbonero A, Ureña-Vacas A, et al. Spontaneous pneumomediastinum in patients with COVID-19: A case series of four patients. [Article in English, Spanish]. Arch Bronconeumol Engl Ed 2020;56(11):754–6. [CrossRef]
[Pubmed]

5.
6.
Alharthy A, Bakirova GH, Bakheet H, et al. COVID-19 with spontaneous pneumothorax, pneumomediastinum, and subcutaneous emphysema in the intensive care unit: Two case reports. J Infect Public Health 2021;14(3):290–2. [CrossRef]
[Pubmed]

7.
Agrawal A, Sen KK, Satapathy G, Sethi HS, Sharawat A, Reddy DS. Spontaneous pneumomediastinum, pneumothorax and subcutaneous emphysema in COVID-19 patients—A case series. Egypt J Radiol Nucl Med 2021;52(1):27. [CrossRef]

8.
Elhakim TS, Abdul HS, Pelaez Romero C, Rodriguez-Fuentes Y. Spontaneous pneumomediastinum, pneumothorax and subcutaneous emphysema in COVID-19 pneumonia: A rare case and literature review. BMJ Case Rep 2020;13(12):e239489. [CrossRef]
[Pubmed]

9.
Quincho-Lopez A, Quincho-Lopez DL, Hurtado-Medina FD. Case report: Pneumothorax and pneumomediastinum as uncommon complications of COVID-19 pneumonia—literature review. Am J Trop Med Hyg 2020;103(3):1170–6. [CrossRef]
[Pubmed]

10.
Sethi SM, Ahmed AS, Hanif S, Aqeel M, Zubairi ABS. Subcutaneous emphysema and pneumomediastinum in patients with COVID-19 disease; case series from a tertiary care hospital in Pakistan. Epidemiol Infect 2021;149:e37. [CrossRef]
[Pubmed]

11.
Shan S, Guangming L, Wei L, Xuedong Y. Spontaneous pneumomediastinum, pneumothorax and subcutaneous emphysema in COVID-19: Case report and literature review. Rev Inst Med Trop São Paulo 2020;62:e76. [CrossRef]
[Pubmed]

12.
Wali A, Rizzo V, Bille A, Routledge T, Chambers AJ. Pneumomediastinum following intubation in COVID-19 patients: A case series. Anaesthesia 2020;75(8):1076–81. [CrossRef]
[Pubmed]

13.
Lemmers DHL, Abu Hilal M, Bnà C, et al. Pneumomediastinum and subcutaneous emphysema in COVID-19: Barotrauma or lung frailty? ERJ Open Res 2020;6(4):00385-2020. [CrossRef]
[Pubmed]

14.
Jiang Y, Guo D, Li C, Chen T, Li R. High-resolution CT features of the COVID-19 infection in Nanchong City: Initial and follow-up changes among different clinical types. Radiol Infect Dis 2020;7(2):71–7. [CrossRef]
[Pubmed]

15.
Lacroix M, Graiess F, Monnier-Cholley L, Arrivé L. SARS-CoV-2 pulmonary infection revealed by subcutaneous emphysema and pneumomediastinum. Intensive Care Med 2020;46(8):1620–1. [CrossRef]
[Pubmed]

16.
Jatoi TA, Khan AA, Mohiuddin O, Choudhry MS, Yasmin F, Jalees S. Spontaneous pneumomediastinum and subcutaneous emphysema in a non-intubated COVID-19 patient: A case report. Pan Afr Med J 2021;38:37. [CrossRef]
[Pubmed]

17.
Diaz A, Patel D, Sayedy N, Anjum F. COVID-19 and spontaneous pneumomediastinum: A case series. Heart Lung 2021;50(2):202–5. [CrossRef]
[Pubmed]

18.
Byun CS, Choi JH, Hwang JJ, Kim DH, Cho HM, Seok JP. Vacuum-assisted closure therapy as an alternative treatment of subcutaneous emphysema. Korean J Thorac Cardiovasc Surg 2013;46(5):383–7. [CrossRef]
[Pubmed]

19.
Manna S, Maron SZ, Cedillo MA, et al. Spontaneous subcutaneous emphysema and pneumomediastinum in non-intubated patients with COVID-19. Clin Imaging 2020;67:207–13. [CrossRef]
[Pubmed]

20.
Gammon RB, Shin MS, Buchalter SE. Pulmonary barotrauma in mechanical ventilation. Patterns and risk factors. Chest 1992;102(2):568–72. [CrossRef]
[Pubmed]

21.
Acute Respiratory Distress Syndrome Network, Brower RG, Matthay MA, et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000;342:1301–8. [CrossRef]
[Pubmed]

SUPPORTING INFORMATION
Author Contributions
Dhouha Ben Braiek - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Imen Mighri - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Hend Zorgati - Acquisition of data, Analysis of data, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Rahma Ben Jazia - Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ameni Kacem - Acquisition of data, Analysis of data, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Jihene Ayachi - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Guarantor of SubmissionThe corresponding author is the guarantor of submission.
Source of SupportNone
Consent StatementWritten informed consent was obtained from the patient for publication of this article.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Conflict of InterestAuthors declare no conflict of interest.
Copyright© 2022 Dhouha Ben Braiek et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.