|

|
 |
|
Case Report
| ||||||
| Limbic encephalitis caused by Epstein–Barr virus: A case report | ||||||
| Zia-ul-haq Peerzada1, Peer Sameer1, Dar Mohammad Salim1, Wani Abdul Haseeb1, Shiekh Yassar1, Shafi Fahad1 | ||||||
|
1MBBS, Radiology resident, Department of Radiodiagnosis and Imaging, Sher-i-Kashmir Institute of Medical Sciences, Srinagar, India.
| ||||||
| ||||||
|
[HTML Abstract]
[PDF Full Text]
[Print This Article]
[Similar article in Pumed] [Similar article in Google Scholar] 
|
| How to cite this article |
| Zia-ul-haq P, Sameer P, Salim DM, Haseeb WA, Yassar S, Fahad S. Limbic encephalitis caused by Epstein–Barr virus: A case report. Int J Case Rep Images 2016;7(6):378–383. |
|
Abstract
|
|
Introduction:
Epstein–Barr virus (EBV) is a double-stranded DNA virus which belongs to the family of herpes viruses. It is a lymphotropic virus affecting the B-lymphocytes and epithelial cells. Neurological manifestations of EBV infection are rare and may or may not co-inside with infectious mononucleosis. A wide range of neurological manifestations of EBV infection have been reported and include, encephalitis, meningitis, cerebellitis, acute disseminated encephalomyelitis (ADEM), transverse myelitis and radiculopathy. Limbic encephalitis caused by EBV is a very rare condition which has been reported in recipients of allogeneic hematopoietic stem cell transplant.
Case Report: We report a case of limbic encephalitis caused by EBV in an immunocompetent 75-year-old woman with no history of infectious mononucleosis and no history of allogeneic hematopoietic stem cell transplantation. Diagnosis of limbic encephalitis was suspected on MR imaging. EBV as a causative agent was confirmed on polymerase chain reaction (PCR) of cerebrospinal fluid sample which demonstrated rising titres of EBV DNA. To the best of our knowledge, this is the first case report of limbic encephalitis caused by EBV in an immunocompetent elderly female with no history of allogeneic stem cell transplantation. Conclusion: Limbic encephalitis is a very rare complication of EBV infection but it should be kept in the list of differential diagnosis while evaluating any patient presenting with clinical features of limbic encephalitis with MRI showing signal abnormalities in the limbic cortex. Establishing an early and accurate diagnosis is the key in management as complete recovery is possible on treatment. | |
|
Keywords:
Epstein–Barr virus, Limbic encephalitis, Magnetic resonance imaging, Polymerase chain reaction
| |
|
Introduction
| ||||||
|
Epstein–Barr virus (EBV) is a double-stranded DNA virus which belongs to the family of herpes viruses [1]. It is a lymphotropic virus affecting the B-lymphocytes and epithelial cells [2]. The manifestations of EBV infection depend on age. Infection in early childhood is usually asymptomatic but if infection occurs in adolescence, it manifests as acute mononucleosis characterized by fever, skin eruption, atypical monocytosis, lymphadenopathy, thrombocytopenia, and liver dysfunction [2] [3] . Neurological manifestations of EBV infection are rare and may or may not co-inside with infectious mononucleosis [3] . A wide range of neurological manifestations of EBV infection have been reported and include, encephalitis, meningitis, cerebellitis, acute disseminated encephalomyelitis (ADEM), transverse myelitis and radiculopathy [2] [3]. Diagnosis of EBV encephalitis can be challenging as clinical features are varied and overlap considerably with other etiologies of encephalitis. Radiologically, EBV encephalitis can be suspected in cases demonstrating T2/FLAIR hyperintensities in cortex, basal ganglia, thalami, brainstem and corpus callosum which may be reversible. Diagnosis can be confirmed by serological evaluation of EBV IgG or IgM levels and by polymerase chain reaction for detection of EBV DNA in cerebrospinal fluid (CSF) and/or serum [1] [2][3][4]. | ||||||
|
Case Report
| ||||||
|
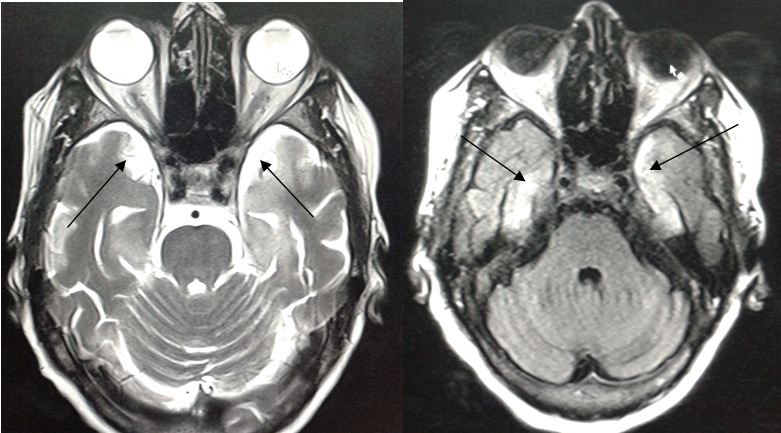
A 75-year-old woman presented to our hospital with complaints of high-grade fever and headache for past few days following which she had few episodes of generalized tonic-clonic seizures, vomiting and confusion. Her family members also reported that she had developed behavioral abnormalities and memory impairment for past few weeks. The patient had anterograde memory loss. Her family members gave a history that she would forget to do simple tasks which she was told to do. She would invariably forget what was said to her and had difficulty in recalling where she had kept a particular object. However, her past memory was intact. She had no past history of any co-morbid illness and was immunocompetent. Her Glasgow Coma Scale (GCS) score was 9/15 (E3V2M4) at the time of presentation and her planters were upgoing bilaterally. Vitals at the time of presentation were: Pulse: 108/min Her pupils were reactive to light laboratory investigations revealed hemoglobin of 9.2g/dl and total leukocyte count of 12000/µl with neutrophils accounting for 82% of the differential count. Kidney and liver function tests were unremarkable. HIV-1, HIV-2, HBsAg tests were negative by card test method. She was a non-alcoholic, non-Smoker. She was non-diabetic. She did not have a history of IV drug abuse or high risk sexual behavior in the past. Non-contrast computed tomography scan of head was grossly unremarkable. MRI was done in our department using Siemens Avanto 1.5 Tesla scanner. On T2/FLAIR images, there was evidence of hyperintense signal from bilateral medial temporal cortices (Figure 1) and multiple hyperintense foci involving bilateral basal ganglia, thalami and white matter (Figure 2). On DWI (Figure 3) and ADC sequences (Figure 4), no evidence of diffusion restriction was seen which ruled out vascular pathology as a cause for the T2/FLAIR hyperintensities. Post Gadolinium sequences showed enhancement in the bilateral medial temporal cortices. Perfusion MR and dynamic MR angiography sequences were also obtained, both of which were unremarkable. The MRI features were similar to that of limbic encephalitis. A qualitative memory assessment, which included tasks like recalling names of objects, season, time of the day, place , repetition of words, etc. was done after she gained consciousness at around seventh day post-admission. She was found to have difficulty in recalling names of the objects shown to her and showed some disorientation to time, place and person. Her remote memory was intact. A mini-mental state examination was not done as the patient was illiterate. A diagnosis of limbic encephalitis was suspected after considering the history of subacute onset of cognitive impairment and anterograde amnesia followed by seizures and signal abnormalities involving bilateral temporal cortices. The patient was empirically started on ampicillin, ceftriaxone and ganciclovir. Diazepam was administered for control of GTCS, but she developed status epilepticus and was shifted to ICU where she was intubated and status epilepticus was managed. CSF examination, done on day-4 post-admission, revealed a cell count of 250 WBCs/µl with 76% polymorphs, glucose of 60 mg/dl, protein of 50 mg/dl. Gram stain and AFB stain were negative and no growth of bacteria was noted on cultures. PCR for herpes simplex and CMV was negative. PCR for Epstein-Barr virus was positive. Repeat CSF examination on day-9 post-admission was again negative for HSV but showed raised titres of EBV DNA. This confirmed the diagnosis of EBV encephalitis. Ampicillin and ceftriaxone were discontinued, while ganciclovir was continued for two weeks. She also received methyl prednisolone pulse therapy for 5 days followed by tapering doses of prednisolone. She improved gradually over a period of few days and by day-10 she was extubated. Repeat CSF examination after two weeks showed 25 WBCs/µl with 90% lymphocytes. Anti-NMDA receptor antibody test on the CSF sample was found to be negative. Other autoantibodies were not tested due to non-availability and financial issues. Her repeat MRI scan after three weeks showed resolution of the hyperintensities. There was no history pointing to an underlying malignancy. Clinical examination was also unremarkable for stigmata of an underlying malignancy. Workup for a possible underlying malignancy in our patient included:
No significant finding, which could suggest a malignancy, was found in these examinations. The patient was subsequently discharged. | ||||||
|
| ||||||
| ||||||
|
| ||||||
| ||||||
|
Discussion
| ||||||
|
The neurological complications of EBV were first reported in 1931 [1]. Fujimoto H et al. found that the CNS manifestations of EBV can be divided into two groups: (i) CNS syndromes associated with primary EBV or reactivated infection, and (ii) those associated with chronic infections. They noted that in the former group diverse CNS syndromes including ADEM can occur, while in the latter chronic or recurrent CNS syndromes can occur [5]. The overall incidence of neurological complications in EBV infection has been reported to be <7% [4]. Doja et al. found that out of 216 children with encephalitis between 1994 and 2003, 21 (9.7%) had EBV infection. Only one patient had classical symptoms of infectious mononucleosis. Fever was noted in 18% while headache was noted in 66% of the cases. Forty-eight percent of the cases had seizures and 57% of the cases had evidence of slow background activity on EEG. The MRI abnormalities were reported in 71% of the cases. Death occurred in two patients while mild deficits were seen in two patients. Sixteen patients were neurologically normal on follow-up. Twelve patients were found to have co-infection with other organisms. Two patients had HSV co-infection, two patients were found to be positive for Mycoplasma pneumoniae, one patient had both HSV and Mycoplasma pneumoniae infection and 1 patient had HHV-7 infection. HHV-6 co-infection was found in four patients [6]. Epstein-Barr virus has predilection to involve the deep nuclei which can explain the T2 and FLAIR hyperintensities in the cortex, thalami, basal ganglia and brain stem [7]. Rarely, extensive white matter involvement and involvement of the splenium of corpus callosum has also been reported [4] [7]. The pattern of distribution of lesions on MRI scan has been found to correlate with pathogenesis and has prognostic significance. Cortical gray matter involvement corresponds to perivascular infiltrates, glial nodules and neuronophagia. The involvement of the splenium of corpus callosum correlates with post-infectious inflammatory demyelination as seen in ADEM and is thought to occur due to the affinity of EBV antigens or induced antibodies for the splenial axons. It has been found that isolated thalamic and brainstem involvement without the involvement of basal ganglia is associated with poor outcome [8]. The sensitivity of MRI scan can be increased by magnetic resonance spectroscopy which helps in differentiating ischemia, demyelination, inflammation, and direct toxic or metabolic injuries, which have overlapping features clinically and on imaging [1]. The role of antivirals in treatment of EBV encephalitis is controversial. Ganciclovir is recommended for broad spectrum anti-DNA virus therapy although it has no specific action against EBV. Moreover, no study has compared ganciclovir with other nucleoside analogs in treatment of EBV-related CNS infection [4] . There is much debate regarding role of methyl-prednisolone in treatment of EBV encephalitis. It is believed to suppress the autoimmune process which could be responsible for the manifestations of EBV encephalitis [4] . In our case, the patient developed EBV encephalitis without associated history of infectious mononucleosis. The clinical features and laboratory investigations were essentially indistinguishable from any other CNS infection. We suspected the diagnosis of limbic encephalitis on the MRI findings of T2/FLAIR hyperintensities involving the medial temporal cortices, basal ganglia, thalami and white matter with no evidence of diffusion restriction. T2 hyperintensities in temporal lobe has a broad differential diagnosis which includes encephalitis, hypoglycemia, toxins, post-ictal or peri-ictal edema, etc. The finding is non-specific for any particular etiology. Both encephalitis and post-ictal edema can show gyriform contrast enhancement and this also does not differentiate the two. Our patient had prolonged seizures prior to MR examination and the T2 hyperintensities could also be due to this reason and reversibility could also be a feature of post-ictal edema. However, post-ictal edema may show diffusion restriction due to cytotoxic edema. But diffusion restriction could also be seen in encephalitis. Our case did not show any diffusion restriction, and we could only rule out an acute vascular insult as a cause of T2 hyperintensities. Our suspicion of EBV encephalitis was based on positive CSF for EBV DNA along with the clinical profile of the patient, both of which favored a possible CNS infection by EBV. However, possibility of seizures contributing to T2 hyperintensities cannot be ruled out on the basis of imaging alone. It may also be possible both the factors contributed to T2 hyperintensities. There was no splenial or brainstem lesion. Our patient required ICU care and intubation for management of her status epilepticus. On establishing correct diagnosis, treatment with ganciclovir and corticosteroids led to complete recovery. Limbic encephalitis is almost always associated with an underlying malignancy [9], but our patient did not have any known underlying malignancy and subsequent workup for malignancy was negative. Workup for autoimmunity was limited to anti-NMDA receptor antibody in CSF which proved to be negative. Limbic encephalitis caused by EBV has been reported in recipients of allogeneic hematopoietic stem cell transplantation [10]. Our patient did not have any such history. Limbic encephalitis syndrome following hematopoietic stem cell transplantation has also been linked to the reactivation of an underlying HHV-6 infection. There may also be a link between HHV-6 and mesial temporal lobe epilepsy in immunocompetent hosts which is under investigation [11]. Thus, our case is a very rare and unique case of limbic encephalitis caused by EBV without any history of malignancy or allogeneic hematopoietic stem cell transplantation. | ||||||
|
Conclusion
| ||||||
|
Limbic encephalitis is a very rare complication of Epstein-Barr virus (EBV) infection but it should be kept in the list of differential diagnosis while evaluating any patient presenting with clinical features of limbic encephalitis with MRI showing signal abnormalities in the limbic cortex. Magnetic resonance imaging scan plays a key role in suggesting the diagnosis and the pattern of lesions on MRI scan can help to predict the prognosis. Diagnosis can be confirmed by serology and PCR. Establishing an early and accurate diagnosis is the key in management as complete recovery is possible on treatment as was evident in our case. | ||||||
|
References
| ||||||
| ||||||
|
[HTML Abstract]
[PDF Full Text]
|
|
Author Contributions
Zia-ul-haq Peerzada – Substantial contributions to conception and design, Acquisition of data, Analysis and interpretation of data, Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published Peer Sameer – Analysis and interpretation of data, Revising it critically for important intellectual content, Final approval of the version to be published Dar Mohammad Salim – Analysis and interpretation of data, Revising it critically for important intellectual content, Final approval of the version to be published Wani Abdul Haseeb – Analysis and interpretation of data, Revising it critically for important intellectual content, Final approval of the version to be published Shiekh Yassar – Analysis and interpretation of data, Revising it critically for important intellectual content, Final approval of the version to be published Shafi Fahad – Analysis and interpretation of data, Revising it critically for important intellectual content, Final approval of the version to be published |
|
Guarantor of submission
The corresponding author is the guarantor of submission. |
|
Source of support
None |
|
Conflict of interest
Authors declare no conflict of interest. |
|
Copyright
© 2016 Zia-ul-haq Peerzada et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information. |
|
|
|
About The Authors
| |||
| |||
| |||
| |||
| |||
| |||
| |||